Question: Use MatLab for coding if needed. The concentration and temperature in a particular non-isothermal batch reactor vary with time according to the following differential equations:
Use MatLab for coding if needed.
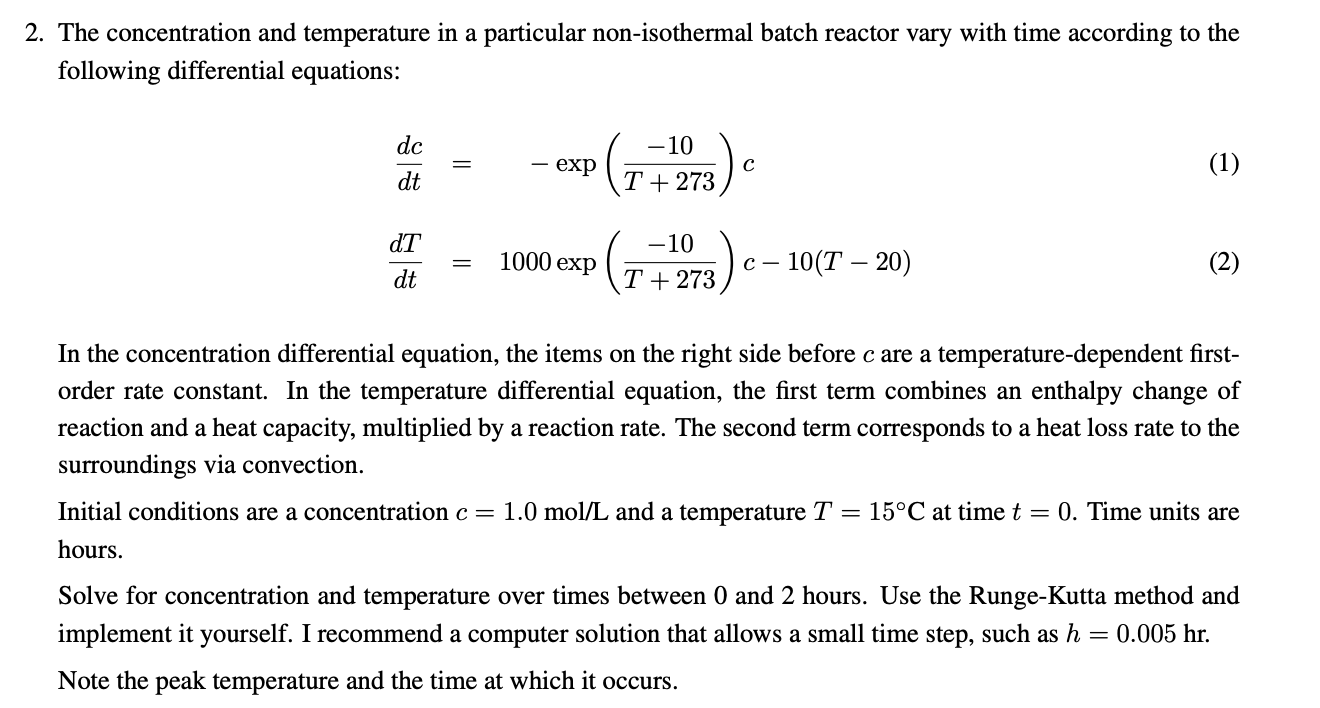
The concentration and temperature in a particular non-isothermal batch reactor vary with time according to the following differential equations: dtdcdtdT=exp(T+27310)c=1000exp(T+27310)c10(T20) In the concentration differential equation, the items on the right side before c are a temperature-dependent firstorder rate constant. In the temperature differential equation, the first term combines an enthalpy change of reaction and a heat capacity, multiplied by a reaction rate. The second term corresponds to a heat loss rate to the surroundings via convection. Initial conditions are a concentration c=1.0mol/L and a temperature T=15C at time t=0. Time units are hours. Solve for concentration and temperature over times between 0 and 2 hours. Use the Runge-Kutta method and implement it yourself. I recommend a computer solution that allows a small time step, such as h=0.005hr. Note the peak temperature and the time at which it occurs. The concentration and temperature in a particular non-isothermal batch reactor vary with time according to the following differential equations: dtdcdtdT=exp(T+27310)c=1000exp(T+27310)c10(T20) In the concentration differential equation, the items on the right side before c are a temperature-dependent firstorder rate constant. In the temperature differential equation, the first term combines an enthalpy change of reaction and a heat capacity, multiplied by a reaction rate. The second term corresponds to a heat loss rate to the surroundings via convection. Initial conditions are a concentration c=1.0mol/L and a temperature T=15C at time t=0. Time units are hours. Solve for concentration and temperature over times between 0 and 2 hours. Use the Runge-Kutta method and implement it yourself. I recommend a computer solution that allows a small time step, such as h=0.005hr. Note the peak temperature and the time at which it occurs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


