Question: use polymath to solve and provide detailed on solution 1. The cracking of gasoil (A) to gasoline (Q) and byproducts (S) follows the multiple reaction
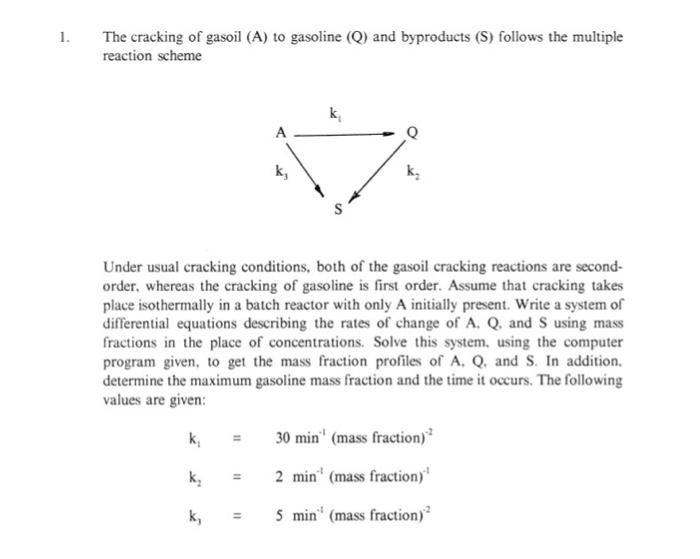


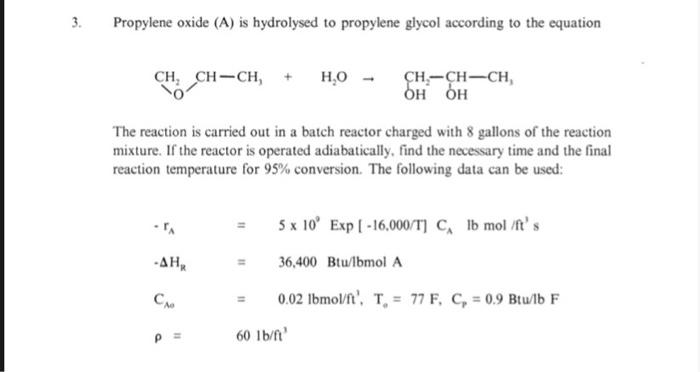
1. The cracking of gasoil (A) to gasoline (Q) and byproducts (S) follows the multiple reaction scheme k A k Under usual cracking conditions, both of the gasoil cracking reactions are second- order, whereas the cracking of gasoline is first order. Assume that cracking takes place isothermally in a batch reactor with only A initially present. Write a system of differential equations describing the rates of change of A, Q. and S using mass fractions in the place of concentrations. Solve this system, using the computer program given, to get the mass fraction profiles of A, Q. and S. In addition, determine the maximum gasoline mass fraction and the time it occurs. The following values are given: 30 min' (mass fraction) 11 kg 11 2 min (mass fraction) k; 11 5 min (mass fraction) The liquid phase reaction P A+B - with a rate expression KCC takes place in a jacketed batch reactor. Using steam as the heating medium, the reaction mixture is heated to help the reaction start. Subsequently, the reaction proceeds adiabatically. During operation, the reaction mixture temperature should not exceed 105C in order to prevent product decomposition. To help keep the reaction temperature below 105C.cooling water can be used in the reactor jacket. For practical reasons, c.g emptying and refilling the reactor jacket, etc. heating and cooling intervals must be separated by at least 10 minutes of adiabatic reaction. For the same reasons it is desirable to operate with as few heating and cooling intervals per batch as possible. (1) Develop a heating/cooling schedule (ii) Calculate and plot the temperature and conversion profiles. DATA m'kmol.s. Tink 2.0 x 10" EXp (-17.500/T) -, 560,000 kJ/kmol A Desired final conversion: X - 0.95 C = 3 kJ/kg. K. P 800 kg V A Initial composition: 50 wt% A. 50% B Molecular weights M = 100, M, = 80 U 2800 W/ mK U = 800 W/ mK T. 20C T= 100C T=15*C, T50c 2 2. The liquid phase reaction of sodium thiosulfate (A) and hydrogen peroxide (B) Nas,o+ 2 H,0, - Products is carried out in a batch reactor. Assume second order kinetics with respect to A. and zero order with respect to B. Determine the necessary time and final reaction mixture temperature for 75% conversion under adiabatic conditions. Use the following data: T 6.853 x 10" Exp(-E/RT) m'mol. s E E 11 76.480 kJ/mol A -, = 548,000 kJ/kmol Cro 11 0.204 kmol/m T. = 25C V 11 2790 cm = 1.05 g/cm ch C 11 4.2 J/g. K 3. Propylene oxide (A) is hydrolysed to propylene glycol according to the equation CH, CH-CH, + ,0 CH-CH-CH OH OH The reaction is carried out in a batch reactor charged with 8 gallons of the reaction mixture. If the reactor is operated adiabatically, find the necessary time and the final reaction temperature for 95% conversion. The following data can be used: -, 5 x 10 Exp | -16.000/T] C, Ib mol it's 36,400 Btu/lbmol A 0.02 Ibmol' T. - 77 F, C, = 0.9 Btulb F 60 1b/ft ) Co P =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


