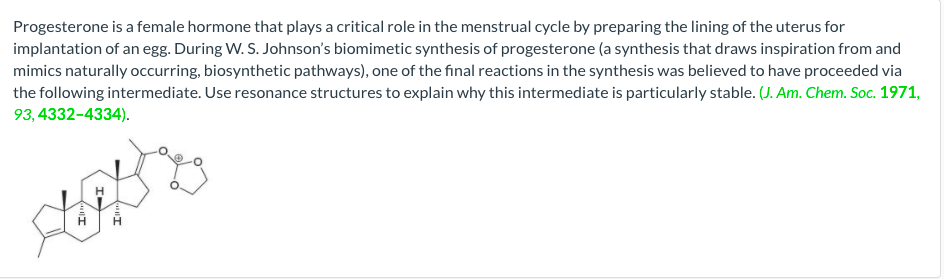
Question: Use resonance structures to identify why this intermediate is particularly stable. Select answer from the options below Highly stabilized by resonance and the positive charge
Use resonance structures to identify why this intermediate is particularly stable.
Select answer from the options below
Highly stabilized by resonance and the positive charge is spread over carbon atom and oxygen atoms.
Highly stabilized by resonance and the positive charge is spread over carbon atoms and oxygen atoms.
Highly stabilized by resonance and the positive charge is spread over carbon atoms and oxygen atom.
Highly stabilized by resonance and the positive charge is spread over carbon atoms and oxygen atoms.
Last saved second ago.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


