Question: use single component phase 52 4. Use the single-component phase diagram below to answer the degree of freedom (DOF) questions. The stars represent points on
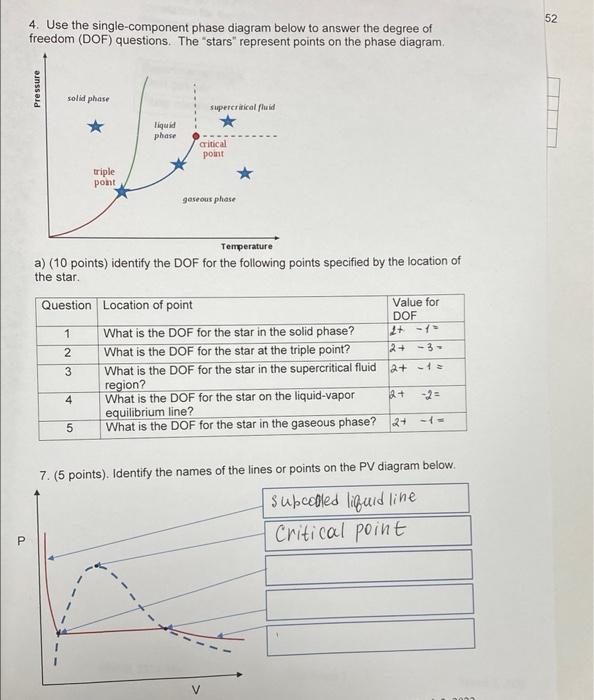

52 4. Use the single-component phase diagram below to answer the degree of freedom (DOF) questions. The stars" represent points on the phase diagram Pressure solid phase supercritical fluid * liquid phase critical pont triple pont gaseous phase Temperature a) (10 points) identify the DOF for the following points specified by the location of the star Question Location of point Value for DOF 1 What is the DOF for the star in the solid phase? 2 What is the DOF for the star at the triple point? 2+ - 3 3 What is the DOF for the star in the supercritical fluid 2+ -+= region? What is the DOF for the star on the liquid-vapor equilibrium line? 5 What is the DOF for the star in the gaseous phase? 27 -- WN 4 2+ 7. (5 points). Identify the names of the lines or points on the PV diagram below. Subcooled liquid line Critical point P V nnnn 5. Setup the calculation for the vapor volume of the methanol gas in Problem 4 by using RKEOS (Redlich/Kwong Equation of State). a. (5 points) Define and provide values for all parameters. b. (5 points) Write out the reduced RKEOS you will use (put in your parameters from step a), and provide the initial value for Zor Vg that will you use. C. (10 points) Perform three hand iterations. How fast does it appear to converge? What is your %-difference after 3 iterations with the experimental value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


