Question: Use the app Note that calculating the % error requires applying the sig fig subtraction Part B: graduated cylinder 1. Use your measurements to calculate
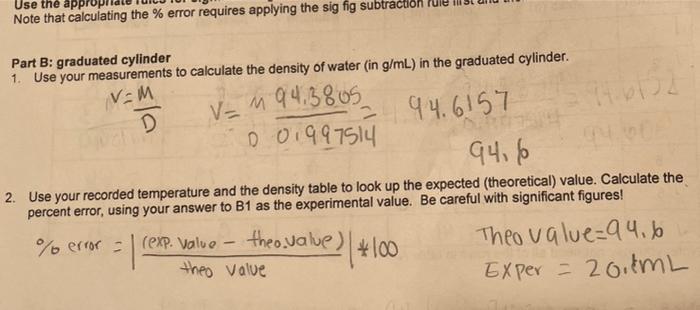

Use the app Note that calculating the % error requires applying the sig fig subtraction Part B: graduated cylinder 1. Use your measurements to calculate the density of water in g/mL) in the graduated cylinder. v=m D V = M 94.3805 2 0 0199714 94.6157 94,6 2. Use your recorded temperature and the density table to look up the expected (theoretical) value. Calculate the percent error, using your answer to B1 as the experimental value. Be careful with significant figures! % error = (exp. Value - theo.value e theo.value) (*100 Theo value=94.6 Exper = 20.8mL theo value Table B Temperature of water Density of water: 0.9 97514 Volume of water (as read from the graduated cylinder) 20.1ML 194.3805 Mass of water in the graduated cylinder
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


