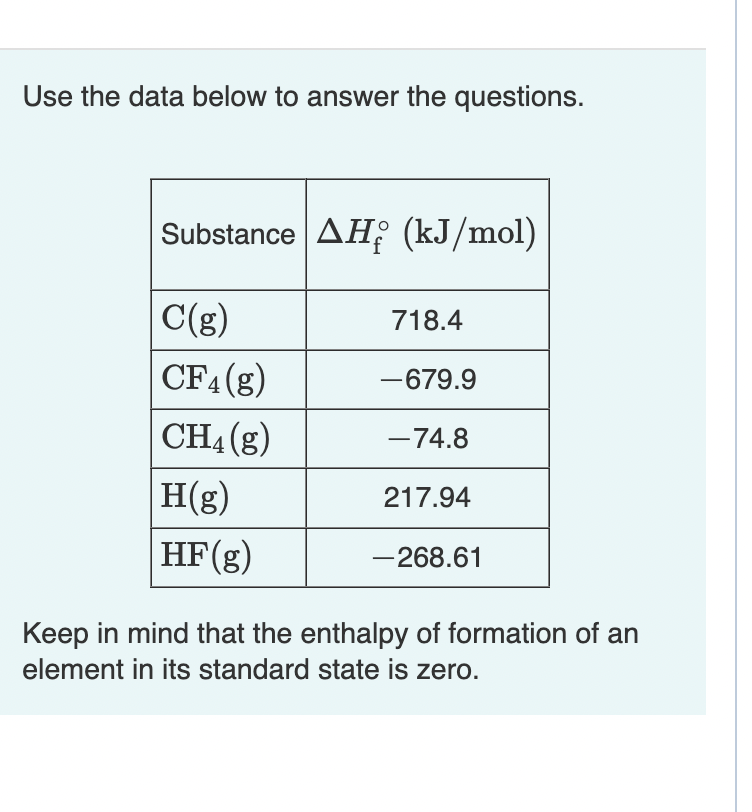
Question: Use the data below to answer the questions. Suppose that 0 . 2 9 0 mol of methane, CH 4 ( g ) is reacted
Use the data below to answer the questions. Suppose that mol
of methane, CHg is reacted with mol of fluorine, Fg
forming CFg and HFg as sole products. Assuming that the reaction occurs at constant pressure, how much heat is released?
Express your answer to three significant figures and include the appropriate units.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


