Question: (Use the data from Turner et al. for all complexes except carbonate - see below) lg1(Ce(CO3)+)=5.33lg2(Ce(CO3)2)=9.24lg1(Eu(CO3)+)=5.75lg2(Eu(CO3)2)=10.11 Based on these calculations, estimate peq for the Ce(IV)/Ce
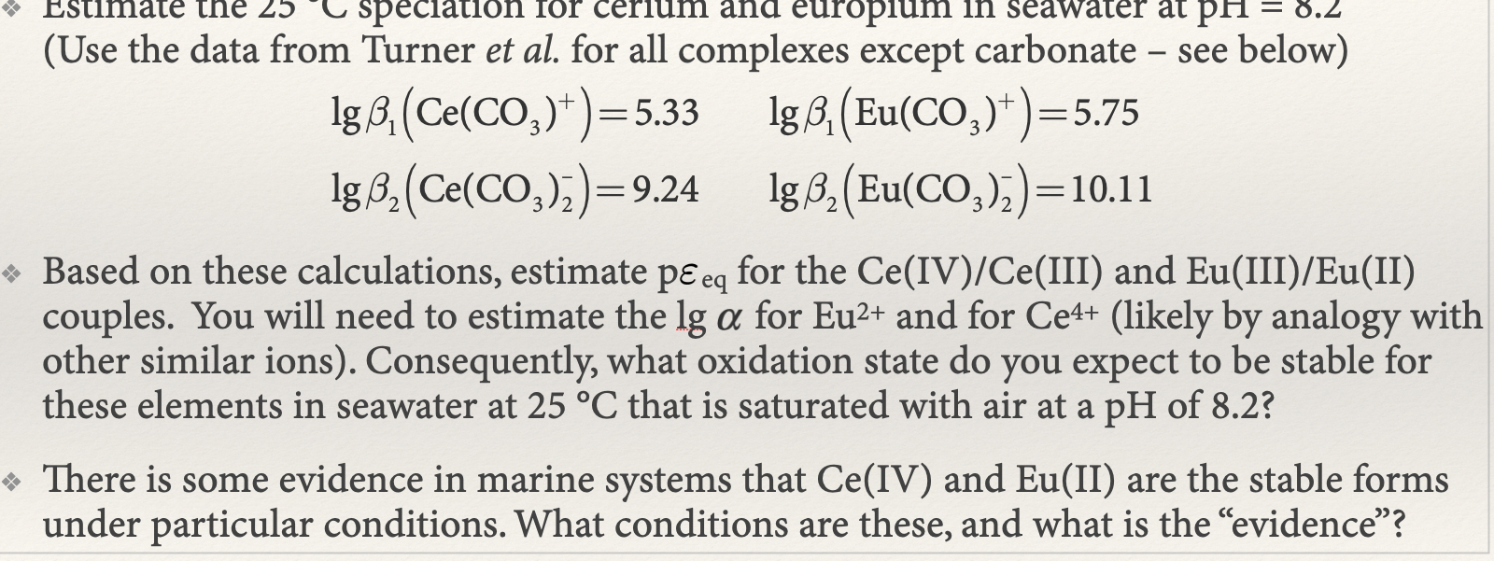
(Use the data from Turner et al. for all complexes except carbonate - see below) lg1(Ce(CO3)+)=5.33lg2(Ce(CO3)2)=9.24lg1(Eu(CO3)+)=5.75lg2(Eu(CO3)2)=10.11 Based on these calculations, estimate peq for the Ce(IV)/Ce (III) and Eu(III)/Eu(II) couples. You will need to estimate the lg for Eu2+ and for Ce4+ (likely by analogy with other similar ions). Consequently, what oxidation state do you expect to be stable for these elements in seawater at 25C that is saturated with air at a pH of 8.2 ? There is some evidence in marine systems that Ce(IV) and Eu(II) are the stable forms under particular conditions. What conditions are these, and what is the "evidence
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


