Question: Use the data given here to calculate the values of AGrxn at 25 C for the reaction described by the equation Compound AG: (kJ/mol) A
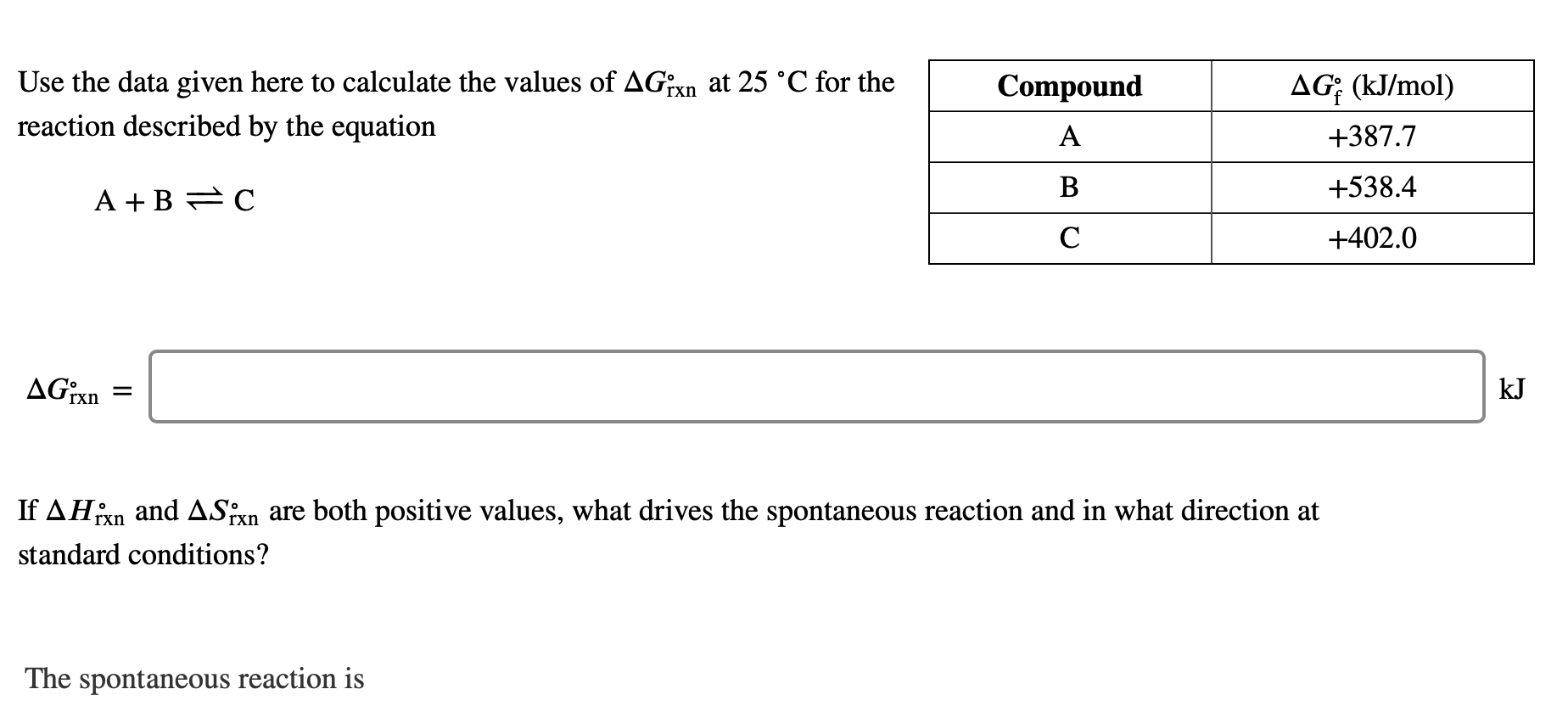

Use the data given here to calculate the values of AGrxn at 25 C for the reaction described by the equation Compound AG: (kJ/mol) A +387.7 B +538.4 A + B FC +402.0 AGixn kJ If AHixn and ASixn are both positive values, what drives the spontaneous reaction and in what direction at standard conditions? The spontaneous reaction is entropy-driven to the right. enthalpy-driven to the right. entropy-driven to the left. enthalpy-driven to the left
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


