Question: Use the figure below for parts D and E. A. What is the difference between a mixture property and a property of mixing? B. Which
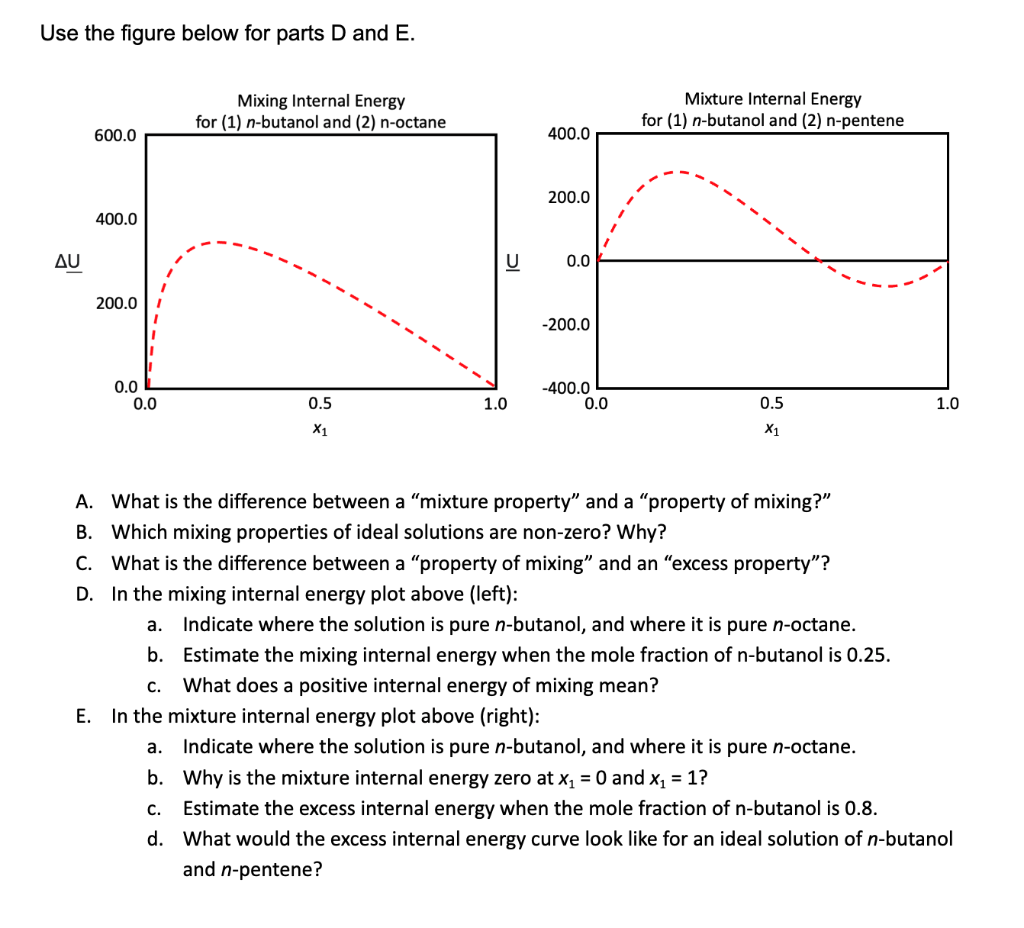
Use the figure below for parts D and E. A. What is the difference between a "mixture property" and a "property of mixing?" B. Which mixing properties of ideal solutions are non-zero? Why? C. What is the difference between a "property of mixing" and an "excess property"? D. In the mixing internal energy plot above (left): a. Indicate where the solution is pure n-butanol, and where it is pure n-octane. b. Estimate the mixing internal energy when the mole fraction of n-butanol is 0.25 . c. What does a positive internal energy of mixing mean? E. In the mixture internal energy plot above (right): a. Indicate where the solution is pure n-butanol, and where it is pure n-octane. b. Why is the mixture internal energy zero at x1=0 and x1=1 ? c. Estimate the excess internal energy when the mole fraction of n-butanol is 0.8 . d. What would the excess internal energy curve look like for an ideal solution of n-butan and n-pentene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


