Question: Use the following information to answer numerical - response question 4 and questions 6 and 7 . Hydrogen cyanide gas, H C N ( g
Use the following information to answer numericalresponse question and questions and
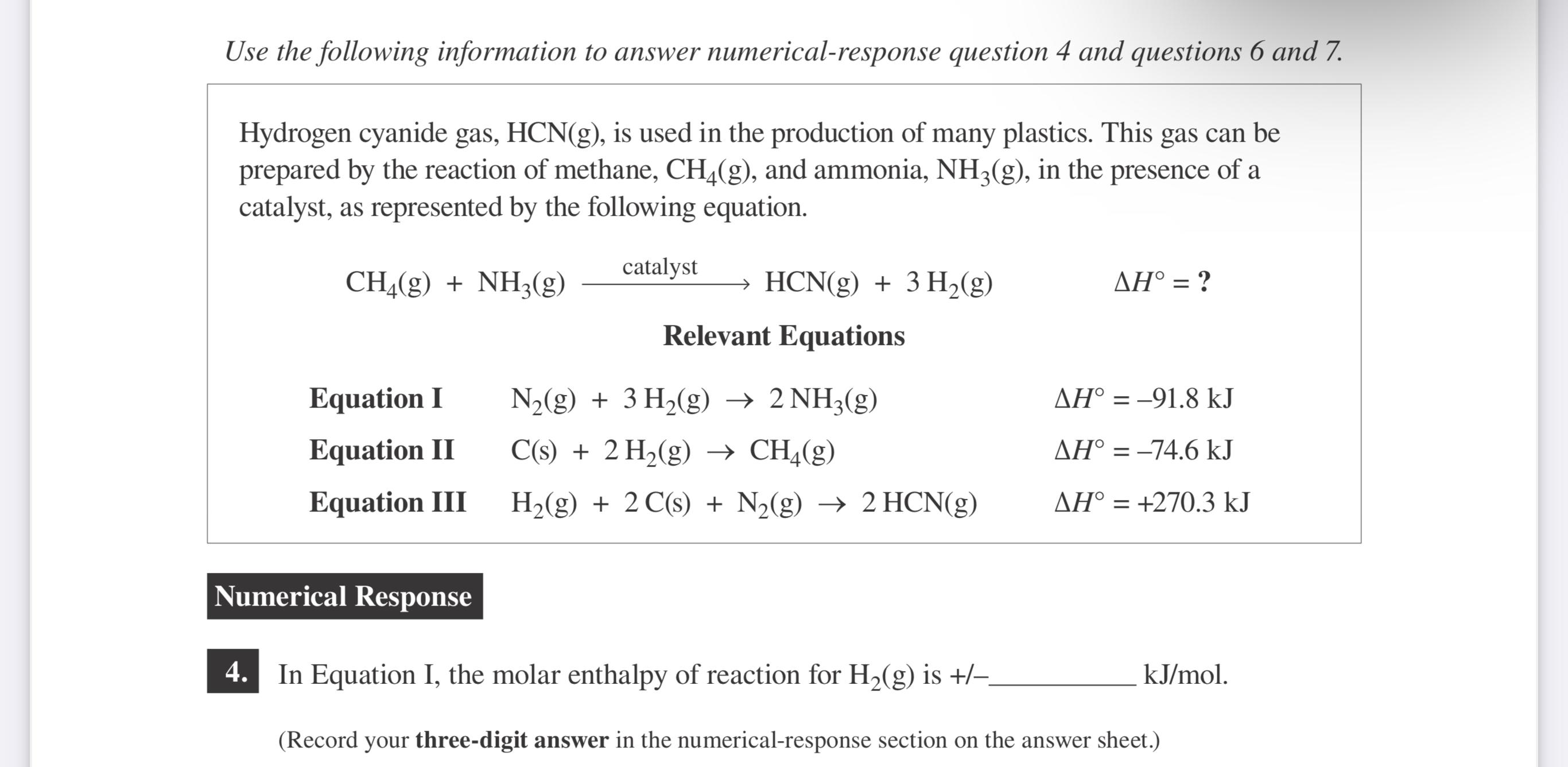
Hydrogen cyanide gas, is used in the production of many plastics. This gas can be prepared by the reaction of methane, and ammonia, in the presence of a catalyst, as represented by the following equation.
catalystHCN
Relevant Equations
Equation I
Equation
Equation III
In Equation I, the molar enthalpy of reaction for is
Record your threedigit answer in the numericalresponse section on the answer sheet.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


