Question: Use the following information to answer the next question. 18mL of stock solution of hydrochloric acid is used to create a 250mL dilute solution with

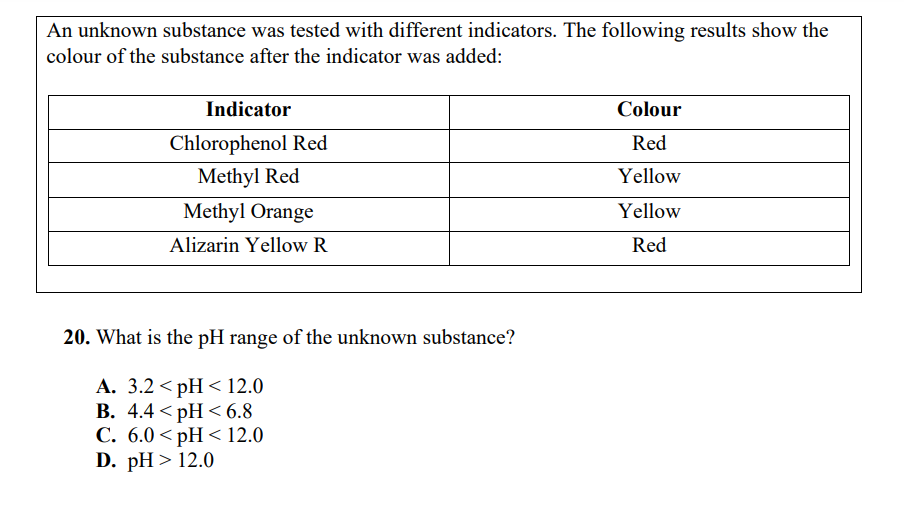
Use the following information to answer the next question. 18mL of stock solution of hydrochloric acid is used to create a 250mL dilute solution with pH of 4.50. 19. What is the concentration of the stock solution? A. 2.31010mol/L B. 4.4104mol/L C. 0.44mol/L D. 9.1mol/L An unknown substance was tested with different indicators. The following results show the colour of the substance after the indicator was added: 20. What is the pH range of the unknown substance? A. 3.2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


