Question: Consider the following infrared spectrum: L00 4000 3000 2000 1000 HAVENUNS ERIl 4. Which of the following functional groups is present in the unknown

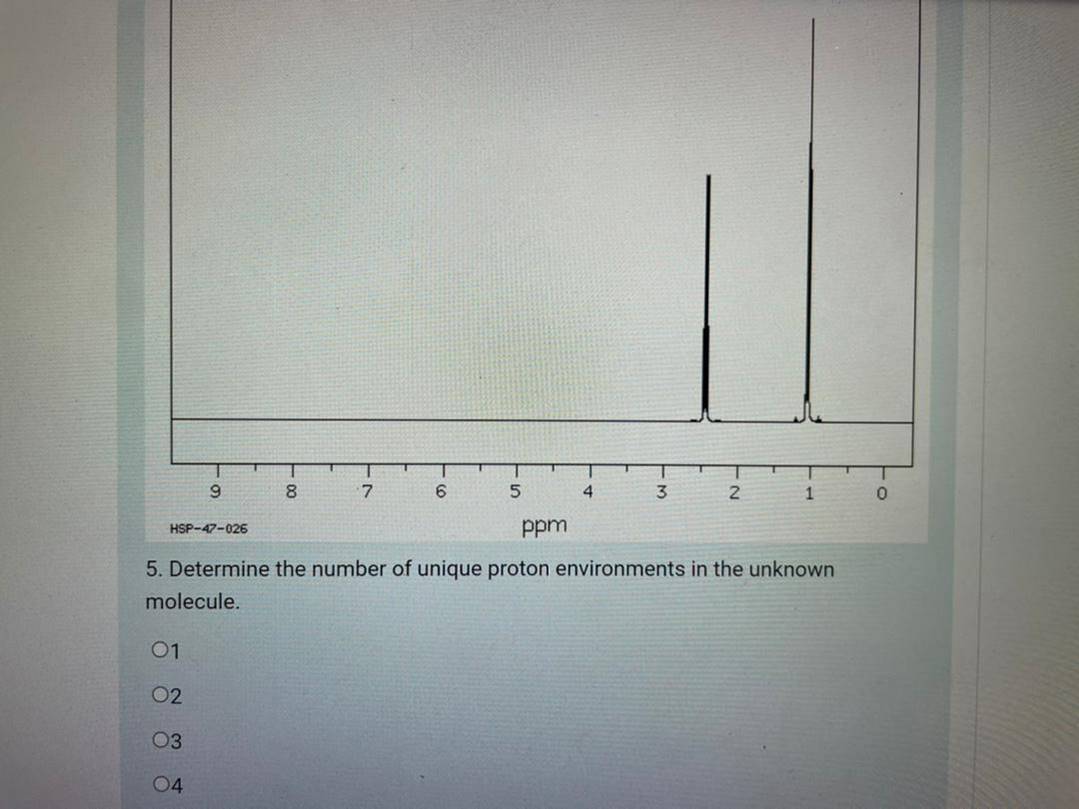

Consider the following infrared spectrum: L00 4000 3000 2000 1000 HAVENUNS ERIl 4. Which of the following functional groups is present in the unknown molecule? OCarbonyl group (C=0) OHydroxyl group (0-H) OAlkene bond (C=C) OAmine group (N-H) TPRNSnETTINCEI 9. 8. 4 2 HSP-47-026 ppm 5. Determine the number of unique proton environments in the unknown molecule. 01 02 03 04 -00 6. The peak at & 2.4 ppm is a triplet, whereas the peak at & 1.0 ppm is a quartet. The peaks show an integration ratio of 3:2 respectively. What does this suggest about the structure of the unknown molecule? ONo structural information can be determined from this. OThe presence of a methyl group (-CH3) OThe presence of an ethyl group (-CH2CH3) OThe presence of a propyl group (-CH2CH2CH3) 7. Identify the unknown molecule from the following choices: Opentanal Opentan-1-ol Opentan-2-one Opentan-3-one HO pentanal pentan-1-ol pentan-2-one pentan-3-one
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Answer Explanation The answer ... View full answer

Get step-by-step solutions from verified subject matter experts


