Question: Use the heating curve below for this question. Which answer provides the best explanation for the difference in time required for boiling as compared to
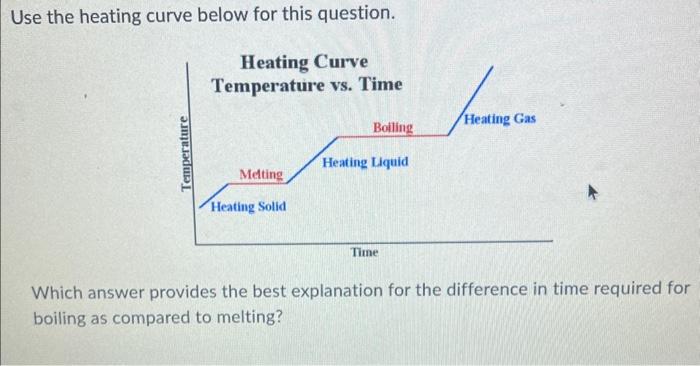

Use the heating curve below for this question. Which answer provides the best explanation for the difference in time required for boiling as compared to melting? Which answer provides the best explanation for the difference in time required for boiling as compared to melting? More time is needed to absorb the greater amount of energy needed to break the intermolecular forces when moving from liquid to gas. When a solid changes to a liquid, more mass is formed; therefore, it takes longer to boil the liquid than to melt the solid. More time is needed to melt than to boil because a lower temperature is required for melting. The two phase changes--melting and boiling--occur at different temperatures. More time is needed to reach the higher temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


