Question: Use the molar bond enthalpy data in the table to estimate the value of AHin for the equation CCI,(g) + 2F,(g) CF_(8) + 2 CI,()
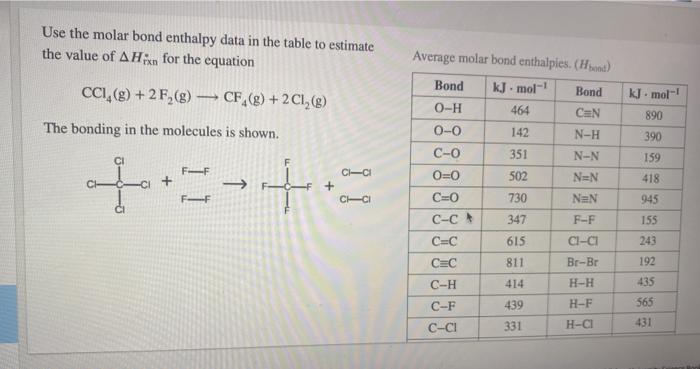
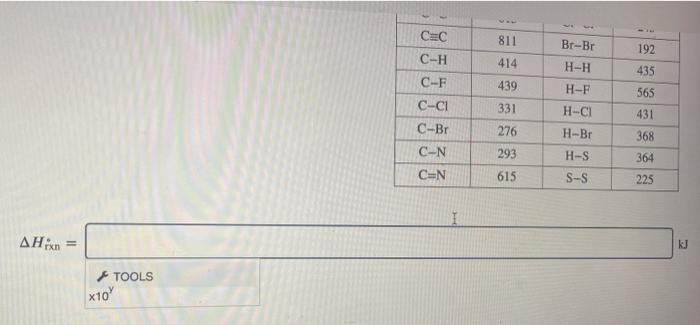
Use the molar bond enthalpy data in the table to estimate the value of AHin for the equation CCI,(g) + 2F,(g) CF_(8) + 2 CI,() kJ. mol-! 890 390 The bonding in the molecules is shown. CI 159 FF CICI 418 + Average molar bond enthalples. (Hoend) Bond kJ. mol-1 Bond 0-H 464 CaN 0-0 142 N-H C-O 351 N-N 0=0 502 NEN C=0 730 NaN C-C 347 F-F C=C 615 CI-CI CEC Br-Br C-H 414 H-H C-F 439 H-F C-CI 331 H-CI FF CCI 945 155 243 811 192 435 565 431 Br-Br 192 H-H CEC C-H C-F C-CI C-Br C-N CEN 811 414 439 331 276 293 615 H-F H-CI H-Br H-S S-S 435 565 431 368 364 225 = kJ TOOLS x10
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


