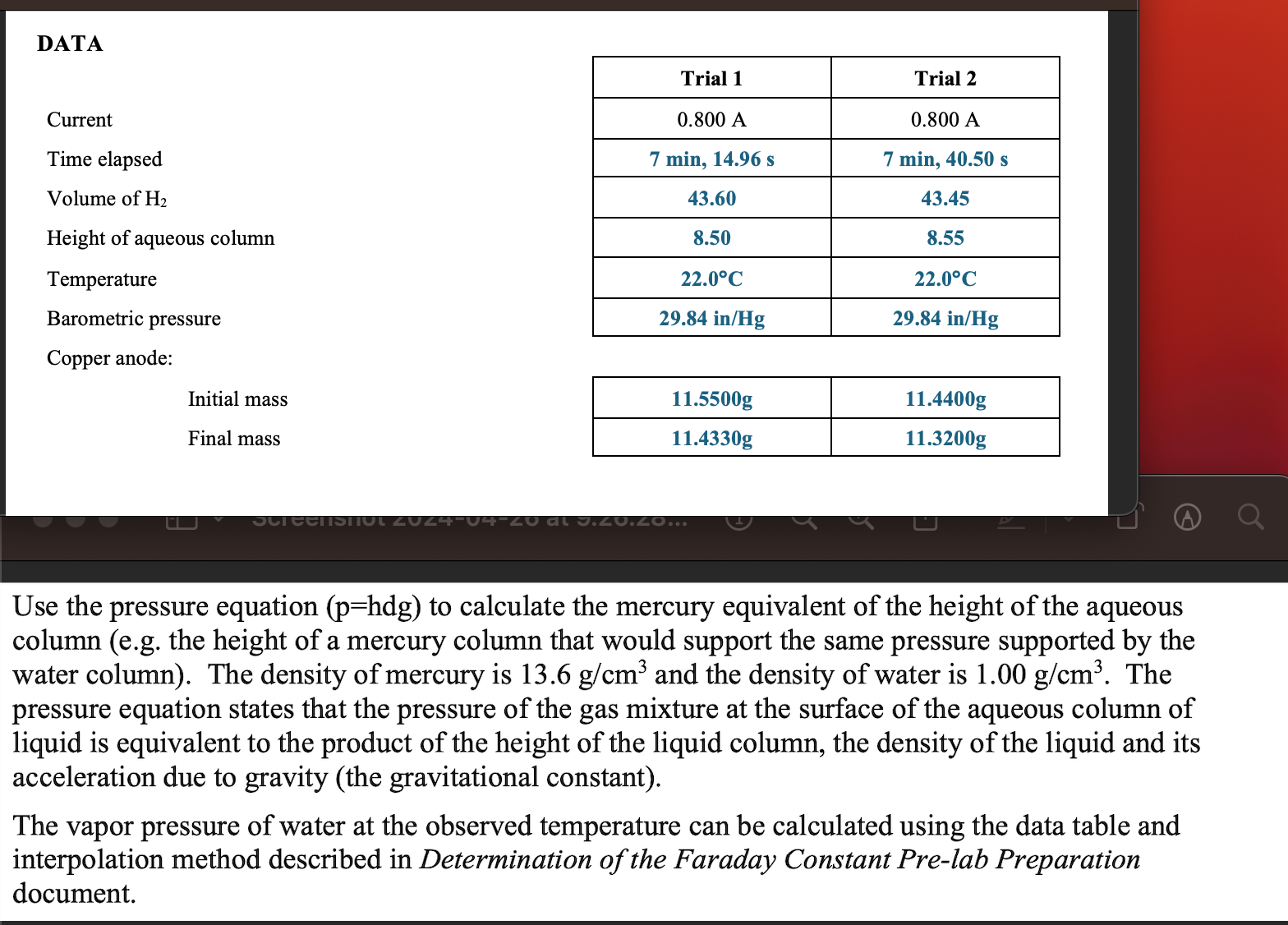
Question: Use the pressure equation ( p = hdg ) to calculate the mercury equivalent of the height of the aqueous column ( e . g
Use the pressure equation phdg to calculate the mercury equivalent of the height of the aqueous column eg the height of a mercury column that would support the same pressure supported by the water column The density of mercury is gcm and the density of water is gcm The pressure equation states that the pressure of the gas mixture at the surface of the aqueous column of liquid is equivalent to the product of the height of the liquid column, the density of the liquid and its acceleration due to gravity the gravitational constant
The vapor pressure of water at the observed temperature can be calculated using the data table and interpolation method described in Determination of the Faraday Constant Prelab Preparation document.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


