Question: 2. Work out the character table for the group C6. Present it in the conventional format, reducing all exponentials to their simplest form. 3.
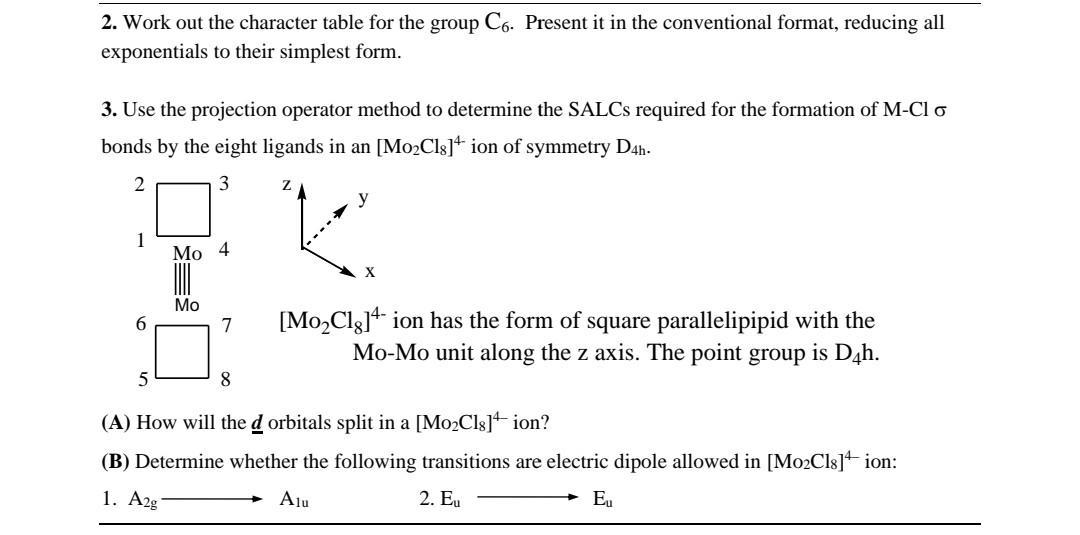
2. Work out the character table for the group C6. Present it in the conventional format, reducing all exponentials to their simplest form. 3. Use the projection operator method to determine the SALCS required for the formation of M-Cl o bonds by the eight ligands in an [Mo2Cls]+ ion of symmetry D4h. 2 3 y 1 Mo 4 X Mo 6. [Mo,Clg]+ ion has the form of square parallelipipid with the Mo-Mo unit along the z axis. The point group is D4h. 7 8 (A) How will the d orbitals split in a [Mo2Cls] ion? (B) Determine whether the following transitions are electric dipole allowed in [Mo2Cls] ion: 1. A2g Alu 2. Eu Eu
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
This is the answer for question no 2 1st question The character table for point group C6 has been wo... View full answer

Get step-by-step solutions from verified subject matter experts


