Question: Use the References to access important values if needed for this question. When the Pb2+ concentration is 1.46M, the observed cell potential at 298K for
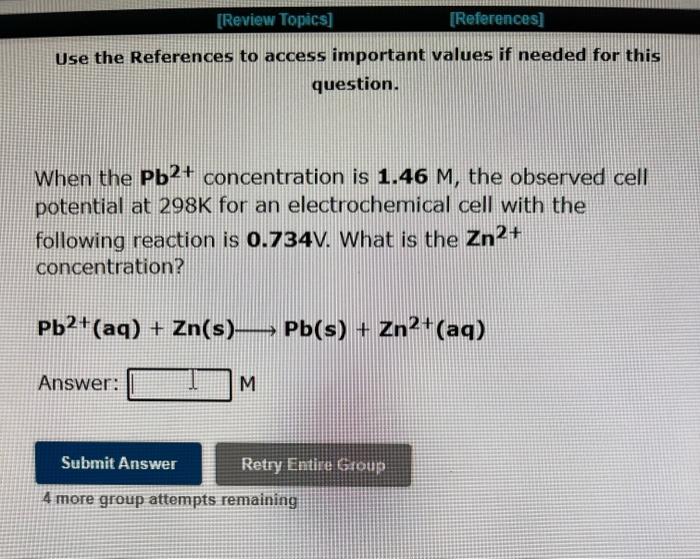
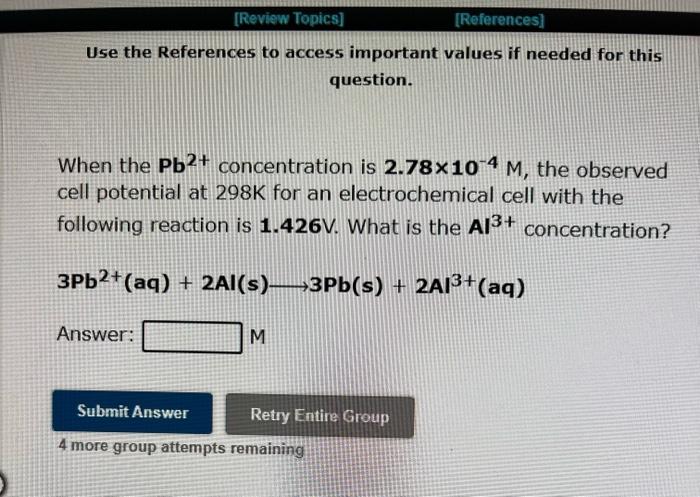
Use the References to access important values if needed for this question. When the Pb2+ concentration is 1.46M, the observed cell potential at 298K for an electrochemical cell with the concentration? Pb2+(aq)+Zn(s)PPb(s)+Zn2+(aq) Answer: M 4 more group attempts remaining Use the References to access important values if needed for this question. When the Pb2+ concentration is 2.78104M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.426V. What is the Al3+ concentration? 3Pb2+(aq)+2Al(s)3Pb(s)+2Al3+(aq) Answer: M 4 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


