Question: Use the References to access important values if needed for this question. a. Use strain energy increments in the OWL Table Reference (see References button,
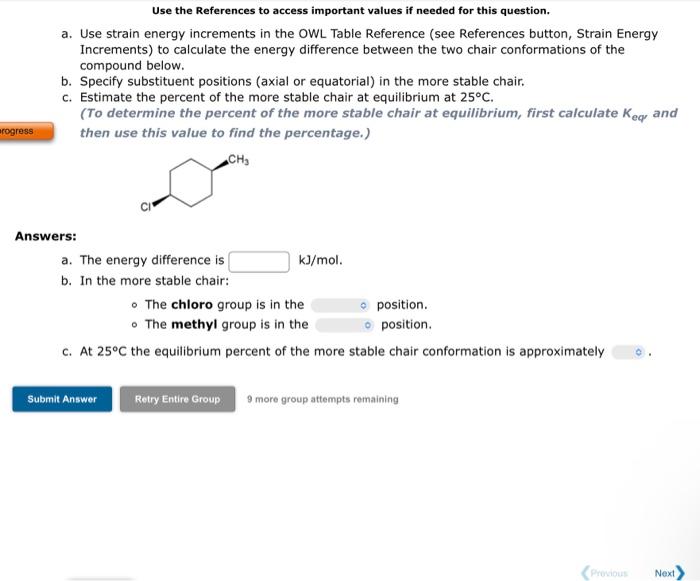
Use the References to access important values if needed for this question. a. Use strain energy increments in the OWL Table Reference (see References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25C. (To determine the percent of the more stable chair at equilibrium, first calculate Keq and then use this value to find the percentage.) Answers: a. The energy difference is kJ/mol. b. In the more stable chair: - The chloro group is in the position. - The methyl group is in the position. c. At 25C the equilibrium percent of the more stable chair conformation is approximately 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


