Question: Use the References to access important values if needed for this question. ICI reacts with H2 in the gas phase according to the following chemical
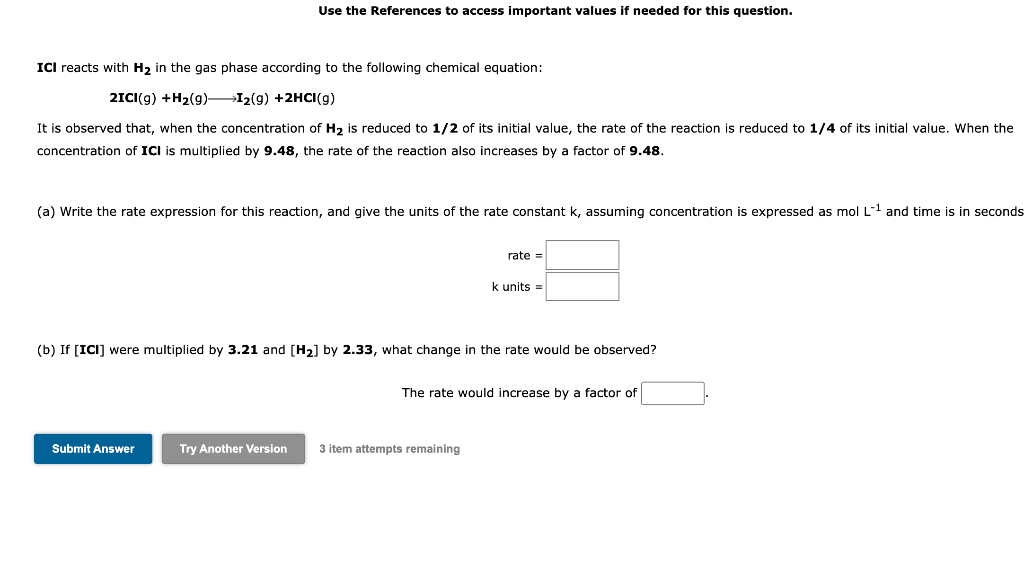
Use the References to access important values if needed for this question. ICI reacts with H2 in the gas phase according to the following chemical equation: 2ICl(g)+H2(g)I2(g)+2HCl(g) It is observed that, when the concentration of H2 is reduced to 1/2 of its initial value, the rate of the reaction is reduced to 1/4 of its initial value. When the concentration of ICl is multiplied by 9.48, the rate of the reaction also increases by a factor of 9.48. (a) Write the rate expression for this reaction, and give the units of the rate constant k,assumingconcentrationisexpressedasmol-1 and time is seconds ratekunits== (b) If [ ICl] were multiplied by 3.21 and [H2] by 2.33, what change in the rate would be observed? The rate would increase by a factor of 3 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


