Question: Use the References to access important values if needed for this question. A 7.500 gram sample of an organic compound containing C,H and O is
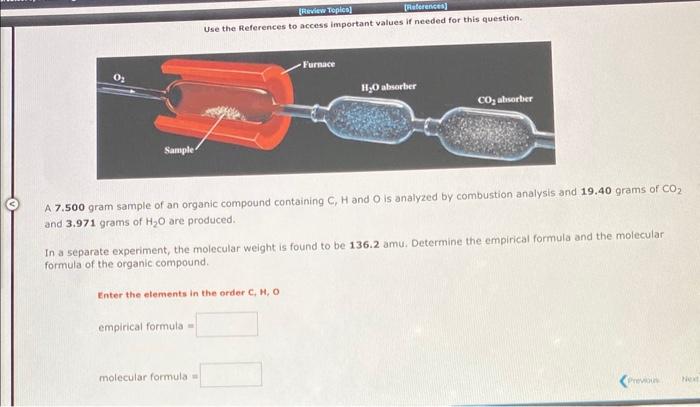
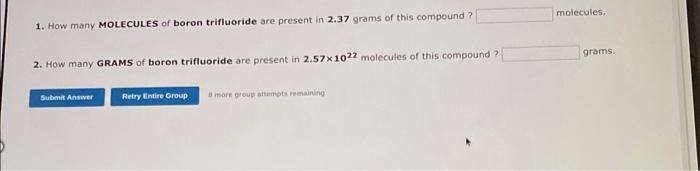
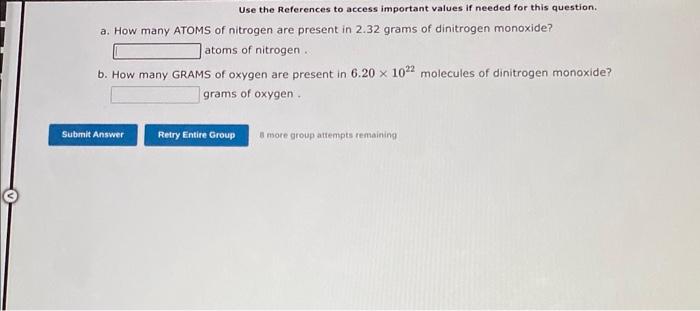
Use the References to access important values if needed for this question. A 7.500 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 19.40 grams of CO2 and 3.971 grams of H2O are produced. In a separate experiment, the molecular weight is found to be 136.2 amu. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C,H,O empirical formula = molecular formula = A 4.378 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 6.527 grams of CO2 and 2.004 grams of H2O are produced. In a separate experiment, the molecular weight is found to be 118.1 amu. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C,H,O embirical formula = molecular formula = 1. How many MOLECULES of boron trifluoride are present in 2.37 grams of this compound? molecules. 2. How many GRAMS of boron trifluoride are present in 2.571022 molecules of this compound? grams: Use the References to access important values if needed for this question. a. How many ATOMS of nitrogen are present in 2.32 grams of dinitrogen monoxide? atoms of nitrogen . b. How many GRAMS of oxygen are present in 6.201022 molecules of dinitrogen monoxide? grams of oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


