Question: Use the References to access important values if needed for this question. A local AM radio station broadcasts at an energy of 5.721031kJ/ photon. (1KHz=103s1)
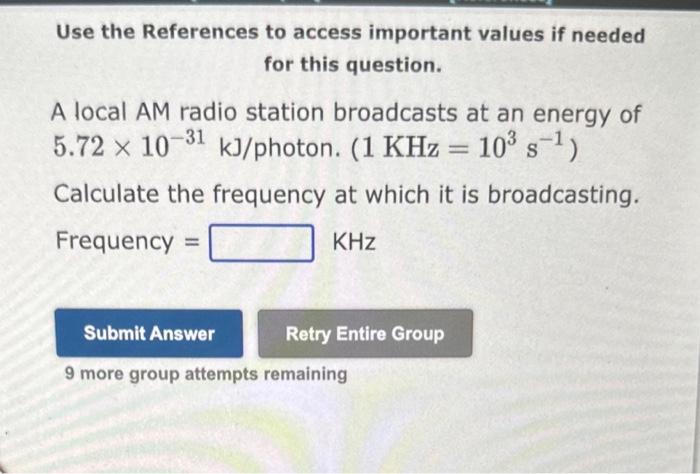
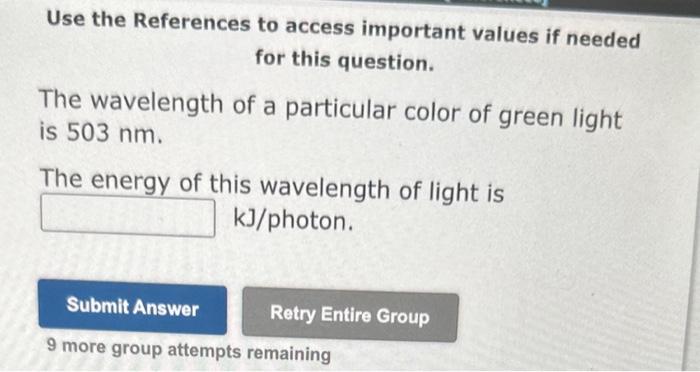
Use the References to access important values if needed for this question. A local AM radio station broadcasts at an energy of 5.721031kJ/ photon. (1KHz=103s1) Calculate the frequency at which it is broadcasting. Frequency =KHz 9 more group attempts remaining Use the References to access important values if needed for this question. The wavelength of a particular color of green light is 503nm. The eneray of this wavelength of light is kJ/ photon. 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


