Question: Use the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for argon: 48 1.00
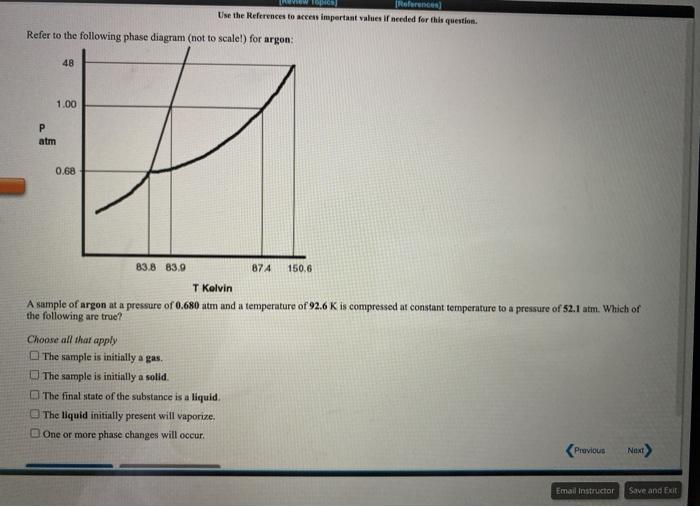
Use the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for argon: 48 1.00 P atm 0.68 83.8 83.9 874 150.6 T Kelvin A sample of argon at a pressure of 0.680 atm and a temperature of 92.6 K is compressed at constant temperature to a pressure of 52.1 atm. Which of the following are true? Choose all that apply The sample is initially a gas The sample is initially a solid The final state of the substance is a liquid The liquid initially present will vaporize. One or more phase changes will occur. Previous Next Email Instructor Save and Exit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


