Question: Use the References to access important values if needed for this question. The hydronium ion concentration in an aqueous solution at 25C is 9.2x 10-2
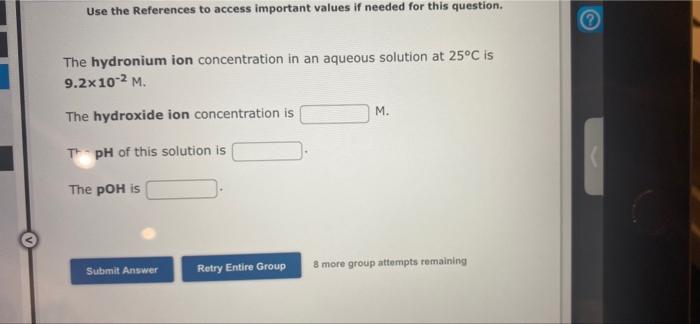
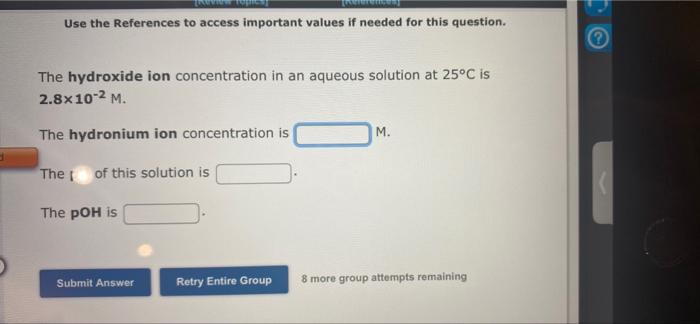
Use the References to access important values if needed for this question. The hydronium ion concentration in an aqueous solution at 25C is 9.2x 10-2 M. M. The hydroxide ion concentration is TpH of this solution is The pOH is Submit Answer Retry Entire Group 8 more group attempts remaining Use the References to access important values if needed for this question. The hydroxide ion concentration in an aqueous solution at 25C is 2.8x10-2 M. The hydronium ion concentration is M. The of this solution is The pOH is Submit Answer Retry Entire Group 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


