Question: Use the relation between the theoretical, the actual, and the % yields (refer to previous prelabs if you forgot the formula) to calculate the %
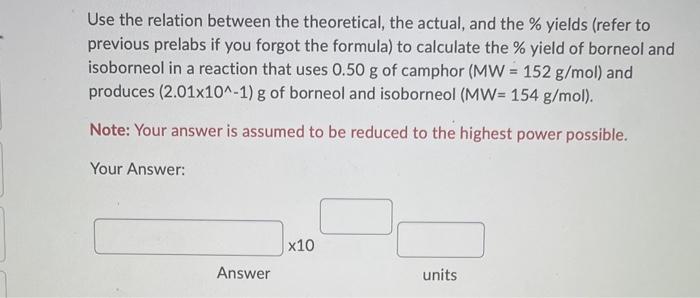
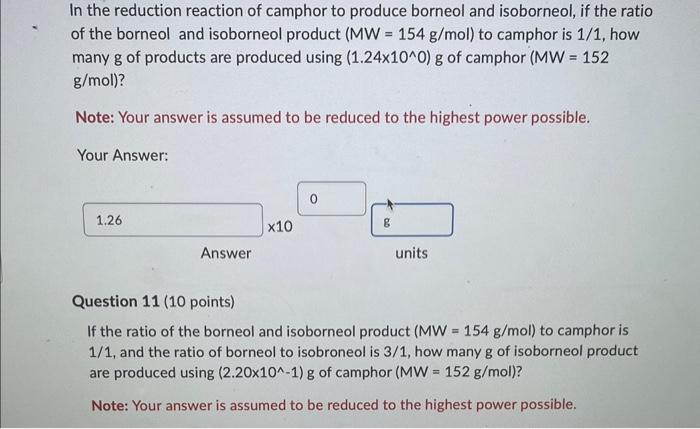
Use the relation between the theoretical, the actual, and the \% yields (refer to previous prelabs if you forgot the formula) to calculate the \% yield of borneol and isoborneol in a reaction that uses 0.50g of camphor (MW=152g/mol) and produces (2.01101)g of borneol and isoborneol (MW=154g/mol). Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: Answer units In the reduction reaction of camphor to produce borneol and isoborneol, if the ratio of the borneol and isoborneol product ( MW=154g/mol ) to camphor is 1/1, how many g of products are produced using (1.24100)g of camphor (MW=152 g/mol) ? Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: Question 11 (10 points) If the ratio of the borneol and isoborneol product (MW=154g/mol) to camphor is 1/1, and the ratio of borneol to isobroneol is 3/1, how many g of isoborneol product are produced using (2.20101)g of camphor (MW=152g/mol) ? Note: Your answer is assumed to be reduced to the highest power possible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


