Question: Use the temperature data you collected to compute the enthalpies for the three reactions: Put your answers in kJ/mol. Use the table of thermodynamic values
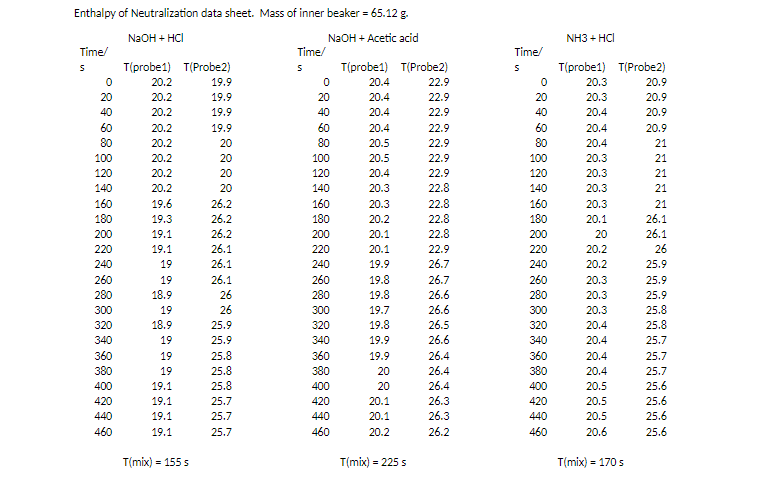
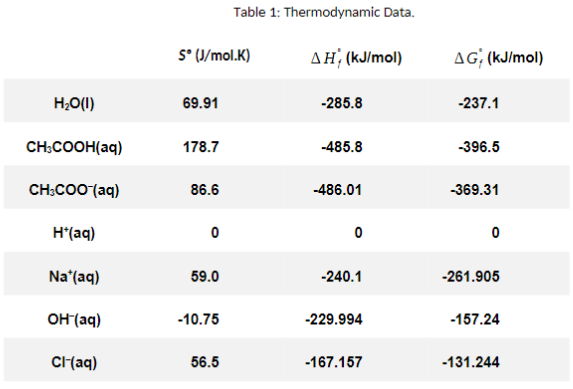
Use the temperature data you collected to compute the enthalpies for the three reactions: Put your answers in kJ/mol.
Use the table of thermodynamic values above (Table 1) along with G=HTS to calculate the entropy and Gibbs Energy changes for the three net ionic equations.
1) Balanced Overall Equation; HCl + NaOH ---> NaCl + H2O
Balanced Net Ionic Equation: H+ + OH- ----> H2O
2) Balanced Overall Equation; HOAc + NaOH ---> NaOAc + H2O
Balanced Net Ionic Equation: H+ + OH- ----> H2O
3) Balanced Overall Equation; HCl + NH3 ---> NH4Cl
Balanced Net Ionic Equation: H+ + NH3 ----> NH4+
NH3 + HCI 22.9 8 8 8 8 8 8888 is N 8 888 22.8 Enthalpy of Neutralization data sheet. Mass of inner beaker = 65.12 g NaOH + HCI NaOH + Acetic acid Time/ Time/ S (probe1) T(Probe 2) S T(probel) (Probe2) 20.2 19.9 0 20.4 22.9 20.2 19.9 20 20.4 22.9 40 20.2 19.9 40 20.4 60 20.2 19.9 60 20.4 22.9 20.2 20 80 20.5 22.9 20.2 20 100 20.5 22.9 20.2 20 120 20.4 22.9 140 20.2 20 140 20.3 26.2 160 20.3 22.8 19.3 26.2 180 20.2 22.8 200 19.1 26.2 200 20.1 220 19.1 26.1 220 20.1 22.9 240 19 26.1 240 19.9 26.7 260 19 26.1 260 19.8 26.7 18.9 26 280 26.6 300 19 26 300 19.7 26.6 320 18.9 25.9 320 19.8 26.5 340 19 25.9 340 19.9 26.6 360 19 25.8 360 19.9 26.4 19 25.8 380 20 26.4 400 19.1 25.8 400 20 420 19.1 25.7 420 20.1 26.3 440 19.1 25.7 440 20.1 26.3 460 19.1 25.7 460 20.2 26.2 19.6 22.8 Time/ S 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 T(probel) T(Probe2) 20.3 20.9 20.3 20.9 20.4 20.9 20.4 20.9 20.4 21 20.3 21 20.3 21 20.3 21 20.3 21 20.1 26.1 20 26.1 20.2 26 20.2 25.9 20.3 25.9 20.3 25.9 20.3 25.8 20.4 25.8 20.4 25.7 20.4 20.4 25.7 20.5 25.6 20.5 25.6 20.5 25.6 20.6 25.6 280 19.8 25.7 380 26.4 T(mix) = 1555 T(mix) = 225 5 T(mix) = 1705 Table 1: Thermodynamic Data. 5 (J/mol.K) AH (kJ/mol) AG(kJ/mol) H20(1) 69.91 -285.8 -237.1 CH3COOH(aq) 178.7 -485.8 -396.5 CH3COOH(aq) 86.6 -486.01 -369.31 H(aq) 0 0 0 Na (aq) 59.0 -240.1 -261.905 OH(aq) -10.75 -229.994 -157.24 Cl(aq) 56.5 -167.157 -131.244
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


