Question: use this example to solve the problem Calculate the change in enthalpy for the following reaction. Be sure your answer has the correct number of
use this example to solve the problem
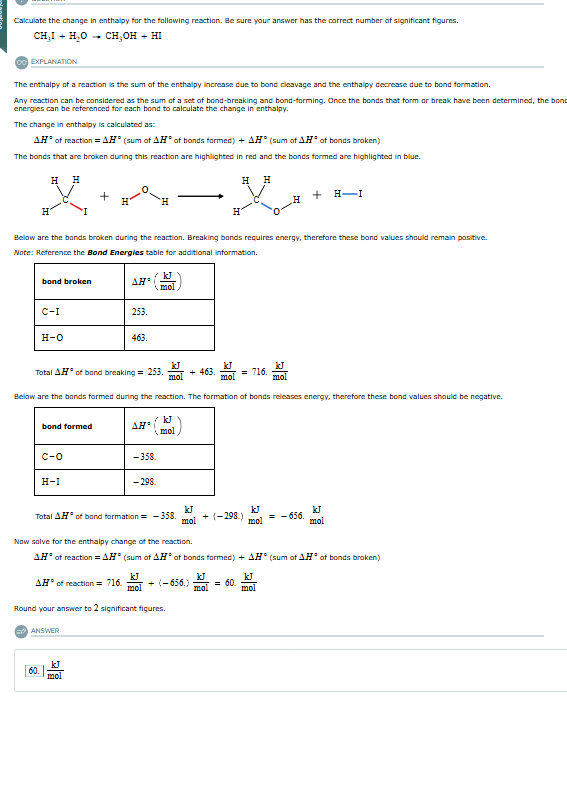 Calculate the change in enthalpy for the following reaction. Be sure your answer has the correct number of significant figures. CH I + H,O - CH.OH + HI EXPLANATION The enthalpy of a reaction is the sum of the enthalpy increase due to bond cleavage and the enthalpy decrease due to bond formation. Any reaction can be considered as the sum of a set of bond-breaking and bond-forming. Once the bonds that form or break have been determined, the bon energies can be referenced for each bond to calculate the change in enthalpy. The change In enthalpy is calculated as: AH' of reaction = A." (sum of AN of bonds formed) + AH" (sum of AN* of bonds broken) The bonds that are broken during this reaction are highlighted in red and the bonds formed are highlighted In blue. + H + H- H H Below are the bonds broken during the reaction. Breaking bonds requires energy, therefore these bond values should remain positive. Note: Reference the Bond Energies table for additional Information. bond broken AH :/ K mol C-I 253 H-0 463. Total .H of bond breaking = 253 KJ mol 7+ 463. KJ mol = 716. - mol Below are the bonds formed during the reaction. The formation of bonds releases energy, therefore these bond values should be negative. band formed AN KI mol C-0 -358 H-I -298 Total A.H" of band formation = - 358. KJ KJ mol + (-292) = - 656. KJ mol " mol Now solve for the enthalpy change of the reaction. AN of reaction = AN" (sum of AN' of bonds formed) + AH" (sum of AN of bonds broken) AH' of reaction = 716. ; KJ KJ mol + (-656.) KJ 'mol = 60 mol Round your answer to 2 significant figures. ANSWER 60. = KJ mol
Calculate the change in enthalpy for the following reaction. Be sure your answer has the correct number of significant figures. CH I + H,O - CH.OH + HI EXPLANATION The enthalpy of a reaction is the sum of the enthalpy increase due to bond cleavage and the enthalpy decrease due to bond formation. Any reaction can be considered as the sum of a set of bond-breaking and bond-forming. Once the bonds that form or break have been determined, the bon energies can be referenced for each bond to calculate the change in enthalpy. The change In enthalpy is calculated as: AH' of reaction = A." (sum of AN of bonds formed) + AH" (sum of AN* of bonds broken) The bonds that are broken during this reaction are highlighted in red and the bonds formed are highlighted In blue. + H + H- H H Below are the bonds broken during the reaction. Breaking bonds requires energy, therefore these bond values should remain positive. Note: Reference the Bond Energies table for additional Information. bond broken AH :/ K mol C-I 253 H-0 463. Total .H of bond breaking = 253 KJ mol 7+ 463. KJ mol = 716. - mol Below are the bonds formed during the reaction. The formation of bonds releases energy, therefore these bond values should be negative. band formed AN KI mol C-0 -358 H-I -298 Total A.H" of band formation = - 358. KJ KJ mol + (-292) = - 656. KJ mol " mol Now solve for the enthalpy change of the reaction. AN of reaction = AN" (sum of AN' of bonds formed) + AH" (sum of AN of bonds broken) AH' of reaction = 716. ; KJ KJ mol + (-656.) KJ 'mol = 60 mol Round your answer to 2 significant figures. ANSWER 60. = KJ mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


