Question: Using 1H NMR, what key features would be used to differentiate ethanal, ethanol and ethanoic acid? Ethanal would give a quartet in the 01.5ppm region,
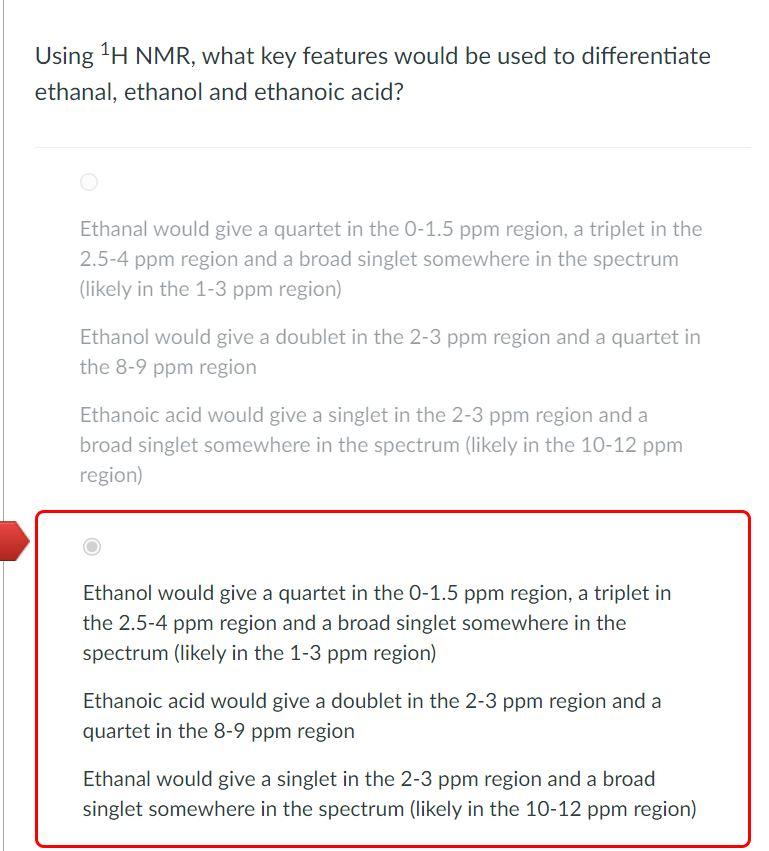

Using 1H NMR, what key features would be used to differentiate ethanal, ethanol and ethanoic acid? Ethanal would give a quartet in the 01.5ppm region, a triplet in the 2.5-4 ppm region and a broad singlet somewhere in the spectrum (likely in the 13ppm region) Ethanol would give a doublet in the 2-3 ppm region and a quartet in the 8-9 ppm region Ethanoic acid would give a singlet in the 23ppm region and a broad singlet somewhere in the spectrum (likely in the 10-12 ppm region) Ethanol would give a quartet in the 0-1.5 ppm region, a triplet in the 2.5-4 ppm region and a broad singlet somewhere in the spectrum (likely in the 1-3 ppm region) Ethanoic acid would give a doublet in the 23ppm region and a quartet in the 8-9 ppm region Ethanal would give a singlet in the 2-3 ppm region and a broad singlet somewhere in the spectrum (likely in the 10-12 ppm region) Ethanoic acid would give a quartet in the 01.5ppm region, a triplet in the 2.5-4 ppm region and a broad singlet somewhere in the spectrum (likely in the 1-3 ppm region) Ethanol would give a doublet in the 2-3 ppm region and a quartet in the 8-9 ppm region Ethanal would give a singlet in the 23ppm region and a broad singlet somewhere in the spectrum (likely in the 10-12 ppm region) Ethanol would give a quartet in the 01.5ppm region, a triplet in the 2.5-4 ppm region and a broad singlet somewhere in the spectrum (likely in the 1-3 ppm region) Ethanal would give a doublet in the 23ppm region and a quartet in the 8-9 ppm region Ethanoic acid would give a singlet in the 23ppm region and a broad singlet somewhere in the spectrum (likely in the 10-12 ppm region)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


