Question: using basic C ++ skills answer the question Ideal gas pressure In thermodynamics, the ideal gas law states PV nRT where P stands for pressure,
using basic C ++ skills answer the question
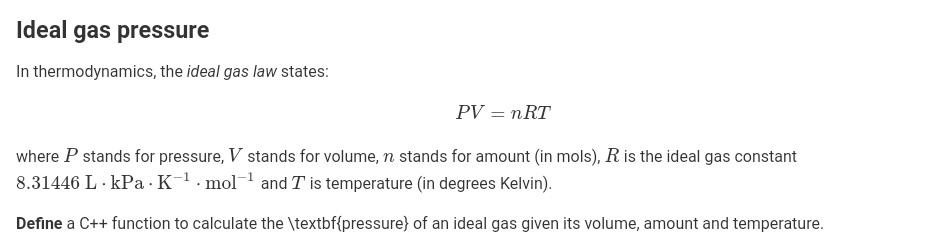
Ideal gas pressure In thermodynamics, the ideal gas law states PV nRT where P stands for pressure, V stands for volume, n stands for amount (in mols), R is the ideal gas constant 8.31446 L kPa K-1 .mol 1 and T is temperature (in degrees Kelvin). Define a C++function to calculate the \textbf (pressure) of an ideal gas given its volume, amount and temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


