Question: Using curved arrows draw at least one resonance structure for each of the followin becies in the space provided below. Note: all the charges are
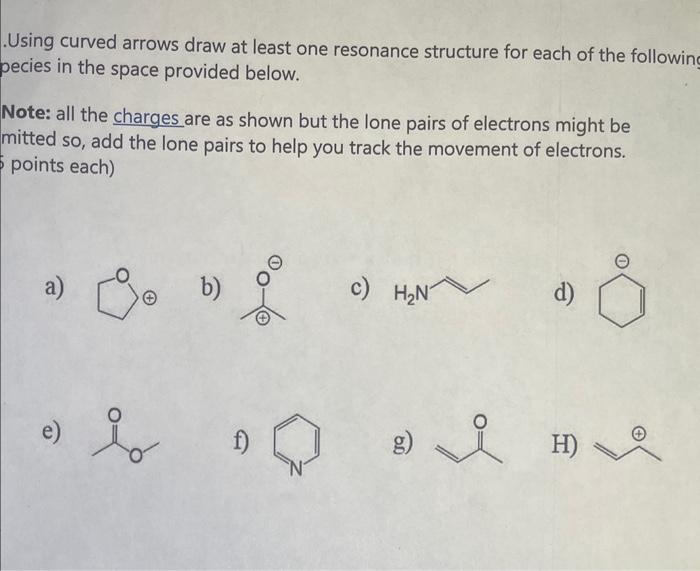
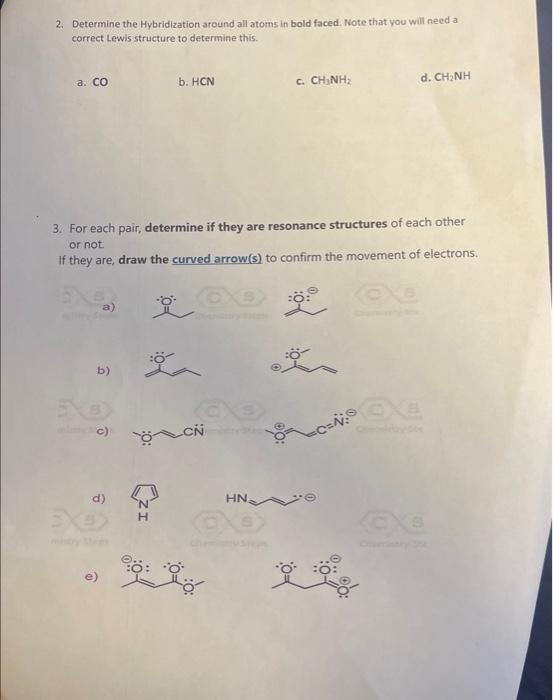
Using curved arrows draw at least one resonance structure for each of the followin becies in the space provided below. Note: all the charges are as shown but the lone pairs of electrons might be nitted so, add the lone pairs to help you track the movement of electrons. points each) a) b) c) d) e) f) g) 2. Determine the Hybridization around all atoms in bold faced. Note that you will need a correct Lewis structure to determine this. a. CO b. HCN c. CH3NH2 d. CH2NH 3. For each pair, determine if they are resonance structures of each other or not If they are, draw the curved arrow(s) to confirm the movement of electrons. a) b) c) d) e)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


