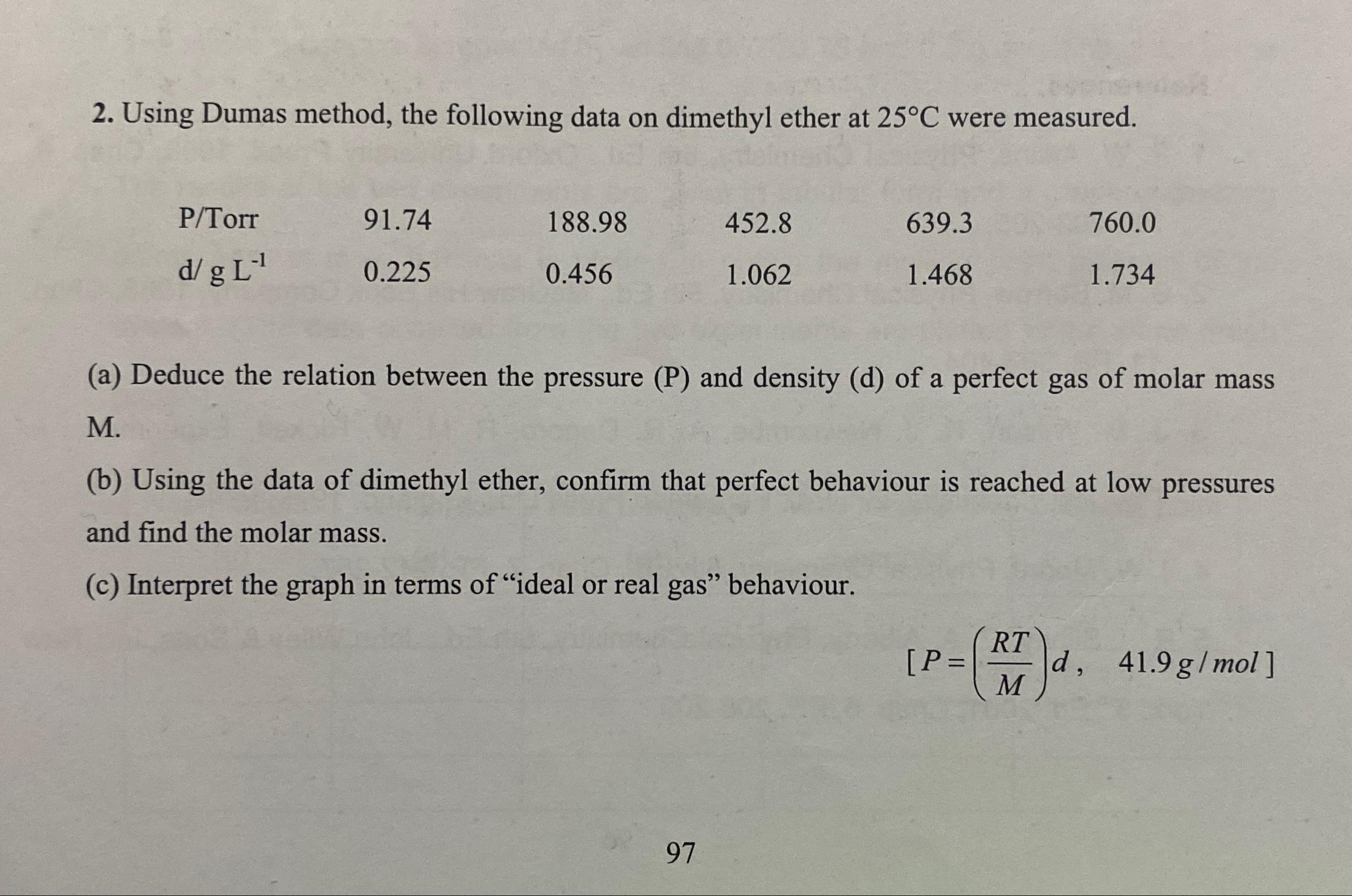
Question: Using Dumas method, the following data on dimethyl ether at 2 5 C were measured. table [ [ P T orr, 9 1 .
Using Dumas method, the following data on dimethyl ether at were measured.
tableorr,
a Deduce the relation between the pressure and density d of a perfect gas of molar mass M
b Using the data of dimethyl ether, confirm that perfect behaviour is reached at low pressures and find the molar mass.
c Interpret the graph in terms of "ideal or real gas" behaviour.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


