Question: Using eBook Table 5.3, how much heat (in Joules) is needed to raise the temperature of 1.1 kgof aluminum from 20 degrees Celsiusto 694 degrees
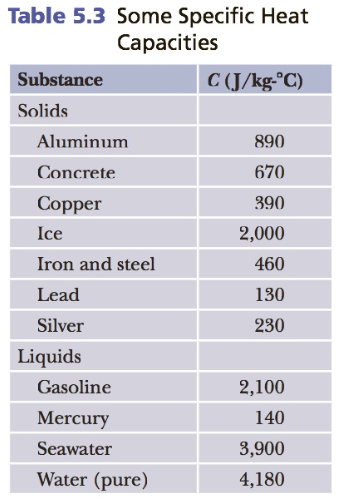
Using eBook Table 5.3, how much heat (in Joules) is needed to raise the temperature of 1.1 kgof aluminum from 20 degrees Celsiusto 694 degrees Celsius?
- Express answers to 3 significant figures unless other instructions are given in the question.
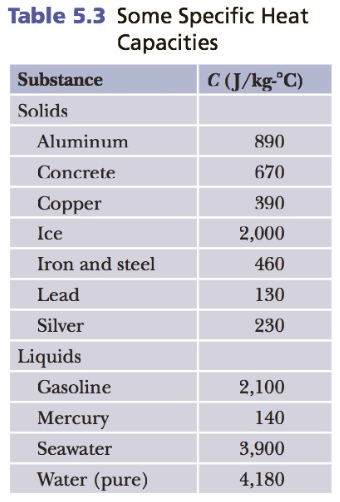
Table 5.3 Some Specific Heat Capacities Substance C (J/kg- C) Solids Aluminum 890 Concrete 670 Copper 390 Ice 2,000 Iron and steel 460 Lead 130 Silver 230 Liquids Gasoline 2,100 Mercury 140 Seawater 3,900 Water (pure) 4,180
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


