Question: Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = x A Preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions
![Using linear regression determine the absorbance/concentration relationship for the dye. [dye] =](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/12/63ae80c8a676f_1672380945335.png)
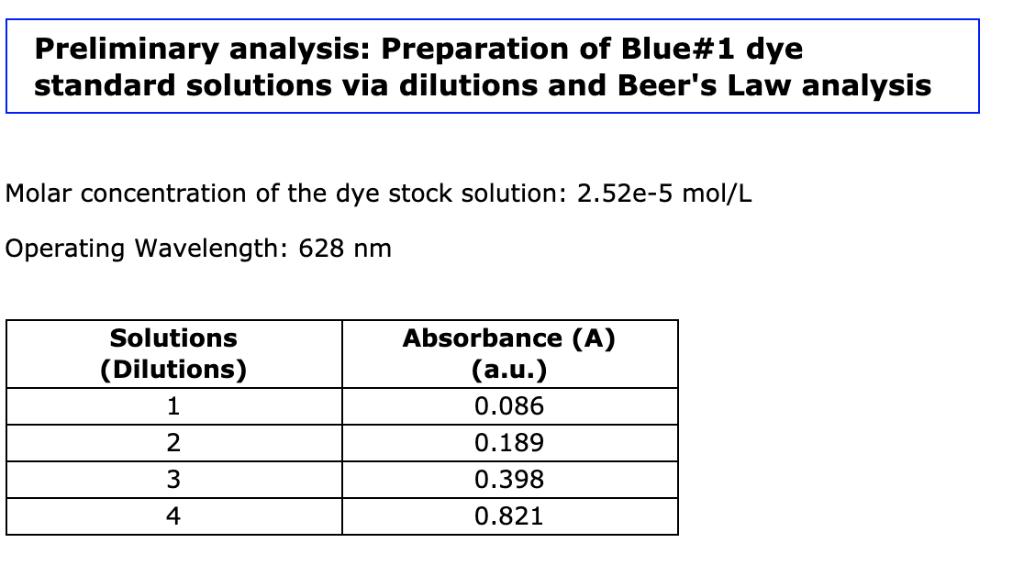

Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = x A Preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions and Beer's Law analysis Molar concentration of the dye stock solution: 2.52e-5 mol/L Operating Wavelength: 628 nm Solutions (Dilutions) 1 2 3 4 Absorbance (A) (a.u.) 0.086 0.189 0.398 0.821 Solution 1: 1.00 mL of Blue#1 Stock Solution and 19.00 mL of distilled water Solution 2: 2.00 mL of Blue#1 Stock Solution and 18.00 mL of distilled water Solution 3: 4.00 mL of Blue#1 Stock Solution and 16.00 mL of distilled water Solution 4: 8.00 mL of Blue#1 Stock Solution and 12.00 mL of distilled water From the preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions and Beer's Law analysis Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = A
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Answer Concentration of initial stock solution 25210 5 molL or M For dilution of Stock solut... View full answer

Get step-by-step solutions from verified subject matter experts


