Question: USING MATLAB PLEASE . THANK YOU IN ADVANCE. If answer is from Polymath I'll automatically give thumbs down P5-21B A microreactor from the MIT group
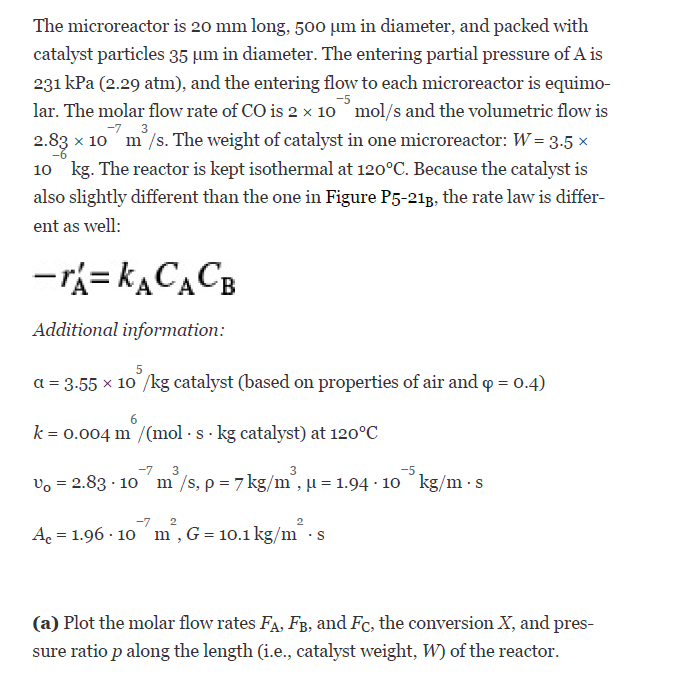
USING MATLAB PLEASE. THANK YOU IN ADVANCE.
If answer is from "Polymath" I'll automatically give thumbs down
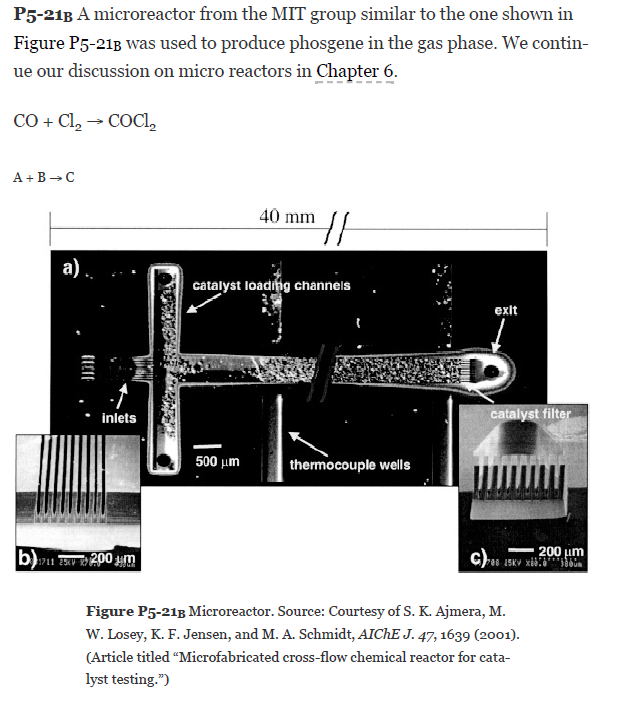
P5-21B A microreactor from the MIT group similar to the one shown in Figure P5-213 was used to produce phosgene in the gas phase. We contin- ue our discussion on micro reactors in Chapter 6. CO + Cl2 COCI, A+B-C 40 mm // a). catalyst loading channels exit Inlets catalyst filter 500 um thermocouple wells bu xxv + 200 um 200 um c) 15 x cm Figure P5-218 Microreactor. Source: Courtesy of S. K. Ajmera, M. W. Losey, K. F. Jensen, and M. A. Schmidt, AICHE J. 47, 1639 (2001). (Article titled Microfabricated cross-flow chemical reactor for cata- lyst testing.") The microreactor is 20 mm long, 500 um in diameter, and packed with catalyst particles 35 um in diameter. The entering partial pressure of A is 231 kPa (2.29 atm), and the entering flow to each microreactor is equimo- lar. The molar flow rate of CO is 2 x 10 mol/s and the volumetric flow is 2.83 x 10 m/s. The weight of catalyst in one microreactor: W=3.5* 10 kg. The reactor is kept isothermal at 120C. Because the catalyst is also slightly different than the one in Figure P5-213, the rate law is differ- ent as well: -rX=KACACB Additional information: a = 3.55 10/kg catalyst (based on properties of air and q = 0.4) k=0.004 m /(mol. s. kg catalyst) at 120C -7 3 3 Vo = 2.83 10 m/s, p7 kg/m, j = 1.94 10 kg/m.s 2 4c = 1.96. 10 m, G = 10.1 kg/ ms (a) Plot the molar flow rates FA, FB, and Fc, the conversion X, and pres- sure ratio p along the length (i.e., catalyst weight, W) of the reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


