Question: using my data, how would u do #3. btw what i did is wrong its not at stp i think. Calculations . Procedure: 1. Put
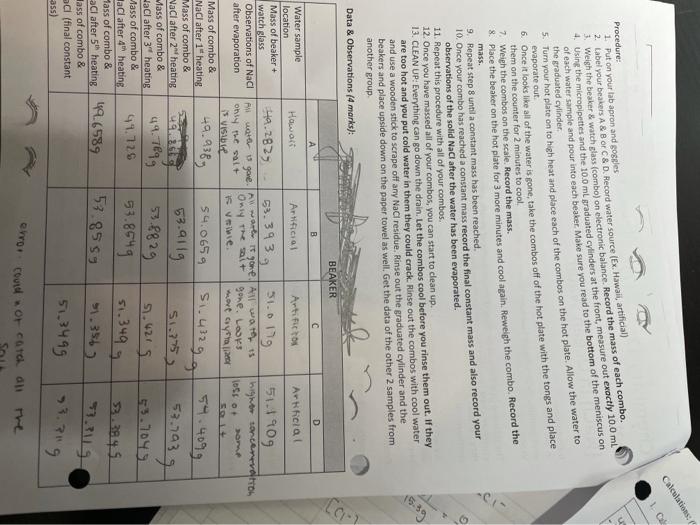
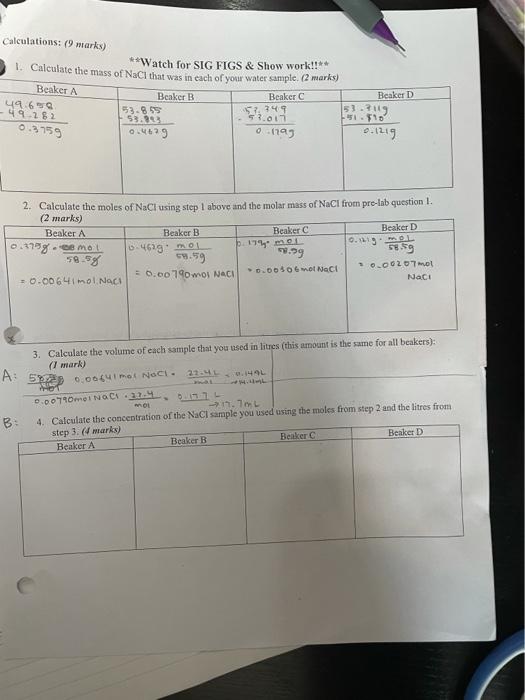
Calculations . Procedure: 1. Put on your tab apron and goggles 2. tabel your beakers A & B C&D. Record water source (Ex. Hawaii, artificial) Weish the besker & watch glass (combo) on electronic balance. Record the mass of each combo. 4 Using the micropipettes and the 10.0 ml graduated cylinders at the front, measure out exactly 10.0 mL the graduated cylinder of each water sample and pour into each beaker. Make sure you read to the bottom of the meniscus on 5. Turn your hot plate on to high heat and place cach of the combos on the hot plate. Allow the water to evaporate 6. Once it looks like all of the water is gone, take the combos off of the hot plate with the tongs and place them on the counter for 2 minutes to cool 7. Weigh the combos on the scale. Record the mass. & Place the beaker on the hot plate for 3 more minutes and cool again. Reweigh the combo. Record the 9. Repeat step 8 until a constant mass has been reached. 10. Once your combo has reached a constant mass record the final constant mass and also record your observations of the solid NaCl after the water has been evaporated. 11. Repeat this procedure with all of your combos. 12. Once you have massed all of your combos, you can start to clean up. 13. CLEAN UP: Everything can go down the drain. Let the combos cool before you rinse them out. If they are too hot and you put cold water in them they could crack. Rinse out the combos with cool water and use a wooden stick to scrape off any NaCl residue. Rinse out the graduated cylinder and the beakers and place upside down on the paper towel as well. Get the data of the other 2 samples from Data & Observations (4 marks): mass. another group BEAKER D Artificial 51190g higher contenevero om 1056 O 51.45gg B C Water sample Hawon Artificial Artific to location Mass of beaker + 49.2829 watch glass 53.3939 51.org Observations of Naci All were gone w wore it gone. Al waters after evaporation only the salt Only the sai+ gone. Los Vie SVIS wort aya Mass of combo & NaCl after 1" heating 49.938g 54.065g Mass of combo & 53.9119 NaCl after heating 51.775 Mass of combo & HaCl after 3" heating 49.7899 51.4219 ass of combo & 41.726 NaCl after heating Mass of combo & aCl after 5" heating 42.6589 lass of combo & acl (final constant 51,3499 ass) 59.4099 53.703 53.704 53 28 29 53.8549 53.8559 51.349 g 31.356) 33.3845 7.png error could not catch all Calculations: (9 marks) Calculate the mass of NaCl that was in cach of your water sample. (2 marks) **Watch for SIG FIGS & Show work!!** Beaker A Beaker B Beaker C Beaker D 49.656 53.2119 53.85 -49.282 11.10 - 53.80 57.00 0.3759 . 0.1193 0.1219 57.749 2. Calculate the moles of NaCl using step 1 above and the molar mass of NaCl from pre-lab question I. (2 marks) Beaker A Beaker B Beaker C Beaker D 0.3758 emot 0.219. MOL 0-461901 17 MOL 59.58 9.59 9.99 5859 0.00$obmol NaCl 0.0020 mol = 0.00 790wol NACI = 0.00641 mol Naci Naci 3. Calculate the volume of each sample that you used in litres (this amount is the same for all beakers): (1 mark) A: 530.00 mo Noct 0.14 PHOT 0.0078 mei NACH OL - 1.7m B: 4. Calculate the concentration of the NaCl sample you used using the moles from step 2 and the litres from step 3. (marks) Beaker A Beaker B Beaker Beaker D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


