Question: Using Octave Chapter 6 textbook- 2, 5, 11, 13 2. Compute the total mass of the following components, using a dot product Component Propellant Steel

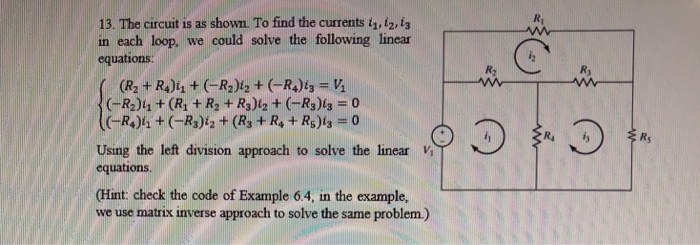
Chapter 6 textbook- 2, 5, 11, 13 2. Compute the total mass of the following components, using a dot product Component Propellant Steel Aluminum Density, g/cm 1.2 7.8 Volume, cm 200 300 5. Bomb calorimeters are used to determine the energy released during chemical reactions. The total heat capacity of a bomb calorimeter is defined as the sum of the product of the mass of each component and the specific heat capacity of each component. That is Tn Where m is the mass of each component, g C is the heat capacity of each component, J/gK and CP is the total heat capacity, J/K Find the total heat capacity of a bomb calorimeter with the following components: Mass, g 250 100 10 A series of experiments were performed with the bomb calorimeter. In each experiment, a different Com Steel Water Aluminum Heat Capacity, J/gK 0.45 4.2 amount of water was used, as shown in the following table Mass of Water, g 110.0 100 1010 98.6 99.4 Calculate the total heat capacity for the calorimeter for each of the experiments 11. Solve the following systems of equations using both the matrix left division and the inverse matrix methods: b) 10x1-7x2 + 0x3-7:-3x1 + 2x2 + 6x3-4 5x1 +32 +5x c) x, + 4x2-23 +x42 6 2xi + 7x2 +x3- 2x4 16 34-10x2-2x3 + 5x.--15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


