Question: Using probabilities to understand ligand-binding in biology (2 + 1 + 2 = 5 points): Imagine a ligand L binding noncovalently to a biological receptor
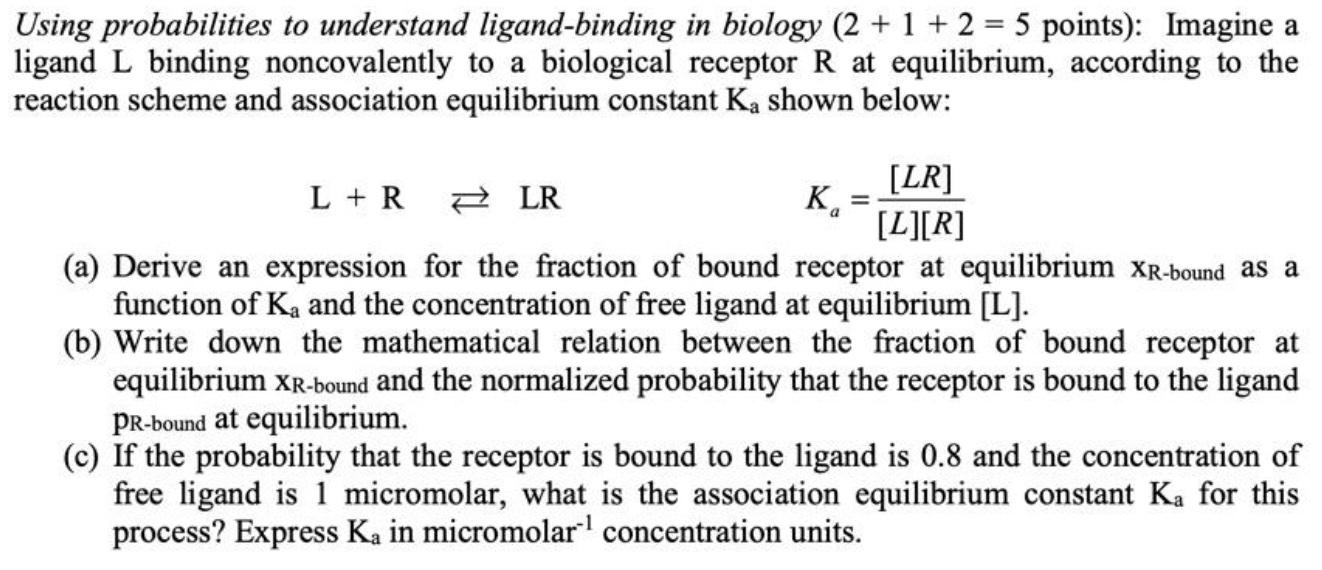
Using probabilities to understand ligand-binding in biology (2 + 1 + 2 = 5 points): Imagine a ligand L binding noncovalently to a biological receptor R at equilibrium, according to the reaction scheme and association equilibrium constant Ka shown below: Ro L + R [LR] LR [L][R] (a) Derive an expression for the fraction of bound receptor at equilibrium XR-bound as a function of Ka and the concentration of free ligand at equilibrium [L]. (b) Write down the mathematical relation between the fraction of bound receptor at equilibrium XR-bound and the normalized probability that the receptor is bound to the ligand PR-bound at equilibrium. (c) If the probability that the receptor is bound to the ligand is 0.8 and the concentration of free ligand is 1 micromolar, what is the association equilibrium constant Ka for this process? Express Ka in micromolar concentration units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


