Question: Using The Activity Series (Table 4.5), Write Balanced NET IONIC Chemical Equations For The Following Reactions. If No Reaction Occurs, Write No Reaction TABLE 4,5
Using The Activity Series (Table 4.5), Write Balanced NET IONIC Chemical Equations For The Following Reactions. If No Reaction Occurs, Write “No Reaction" TABLE 4,5 . Activity Series Of Metals In Aqueous SC 2. Potassium Metal Is Added To A Solution Of Metal Oxidation Reaction Copper(II) Nitrate Lithium Li() - "() + Potassium KO) K() Barium Ba(S) Bo?"(-) +
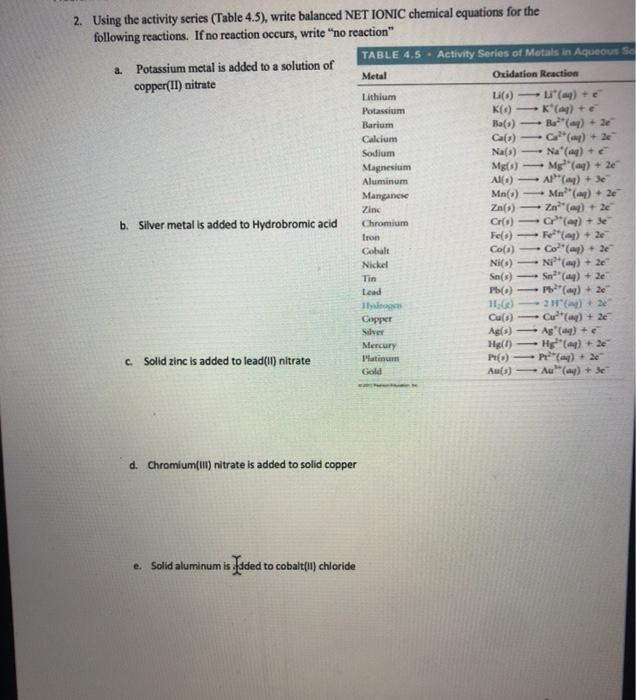
2. Using the activity series (Table 4.5), write balanced NET IONIC chemical equations for the following reactions. If no reaction occurs, write "no reaction" a. Potassium metal is added to a solution of copper(II) nitrate TABLE 4.5 Activity Series of Metals in Aqueous So b. Silver metal is added to Hydrobromic acid Metal Oxidation Reaction Lithium Li(s) Li(aq) + e Potassium K() - K*(aq) + e Barium Ba(3) Ba (aq) + 2e Calcium Ca(s)-> Ca (my) + 2e Sodium Na(s) Na' (aq) + e Magnesium Mg(s) Mg (aq) + 2e Aluminum Al() Al(aq) + 3e Manganese Mn(s) Zinc Chromium Iron Cobalt Co(s) Zn() Ma (aq) + 2e Zn (aq) + 2e Cr(s)-> Cr" (aq) + 3e Fe(s)-> Fe(aq) + 2e Co(aq) + 2e Nickel Ni(s) Ni (aq) + 2e Tin Sn(s) Lead c. Solid zinc is added to lead(II) nitrate Hydrogen Copper Silver Sn" (aq) + 2e Pb(s)-> Pb(aq) + 2e 11,(x) 211(a)+2e Cu(s) Cu (mg) + 2e Ag(s) Ag (aq) + e Mercury Hg() Hg (aq) + 2e Platinum Gold Pt(s) P(aq) +20 Au(s) Au (aq) + 30 d. Chromium(III) nitrate is added to solid copper e. Solid aluminum is added to cobalt(II) chloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


