Question: Using the average bond enthalpy data given in the table below, calculate the mass of compound 1 required to heat 100 g of water
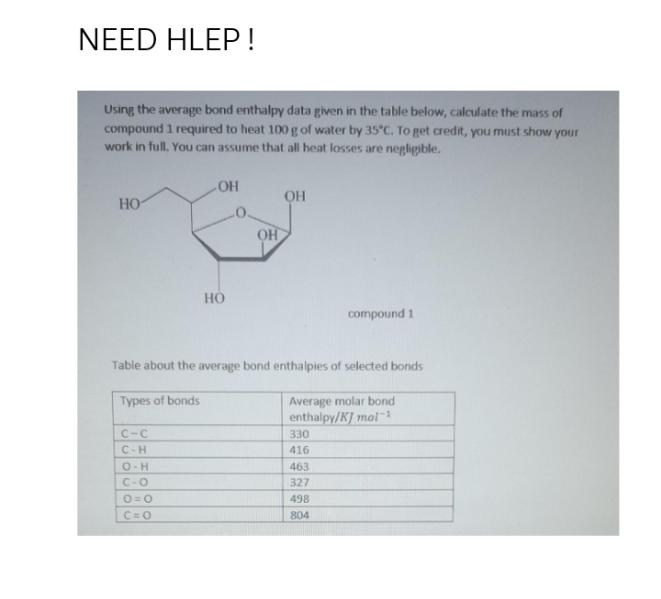
Using the average bond enthalpy data given in the table below, calculate the mass of compound 1 required to heat 100 g of water by 35C. To get credit, you must show your work in full. You can assume that all heat losses are negligible. HO C-C C-H OH O-H C-O O=O C=O HO OH OH Table about the average bond enthalpies of selected bonds Types of bonds Average molar bond enthalpy/KJ mol- 330 416 compound 1 463 327 498 804
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


