Question: Using the data below, derive the equation showing the reaction equilibrium constant as a function of temperature. HO(g) + CH4(g) CH5OH(g) Use y the following
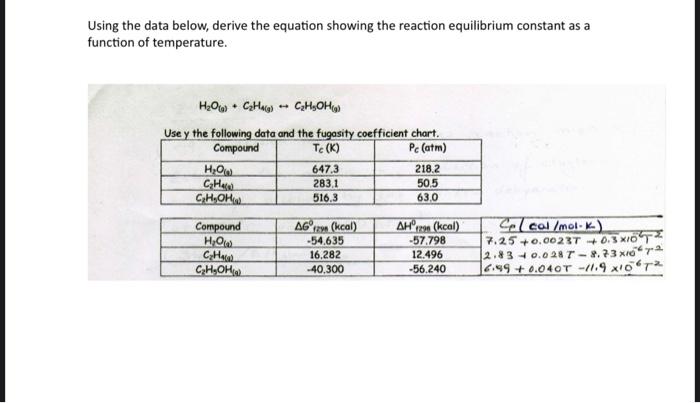
Using the data below, derive the equation showing the reaction equilibrium constant as a function of temperature. H2O(g)+C2H4(g)C2H5OH(g) Use v the followina data and the fuaosity coefficient chart
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


