Question: Using the data below (tabulated at 25C ), what is the vapor pressure, in kilopascals (kPa), of a solution formed by mixing 8.96mol of iodomethane
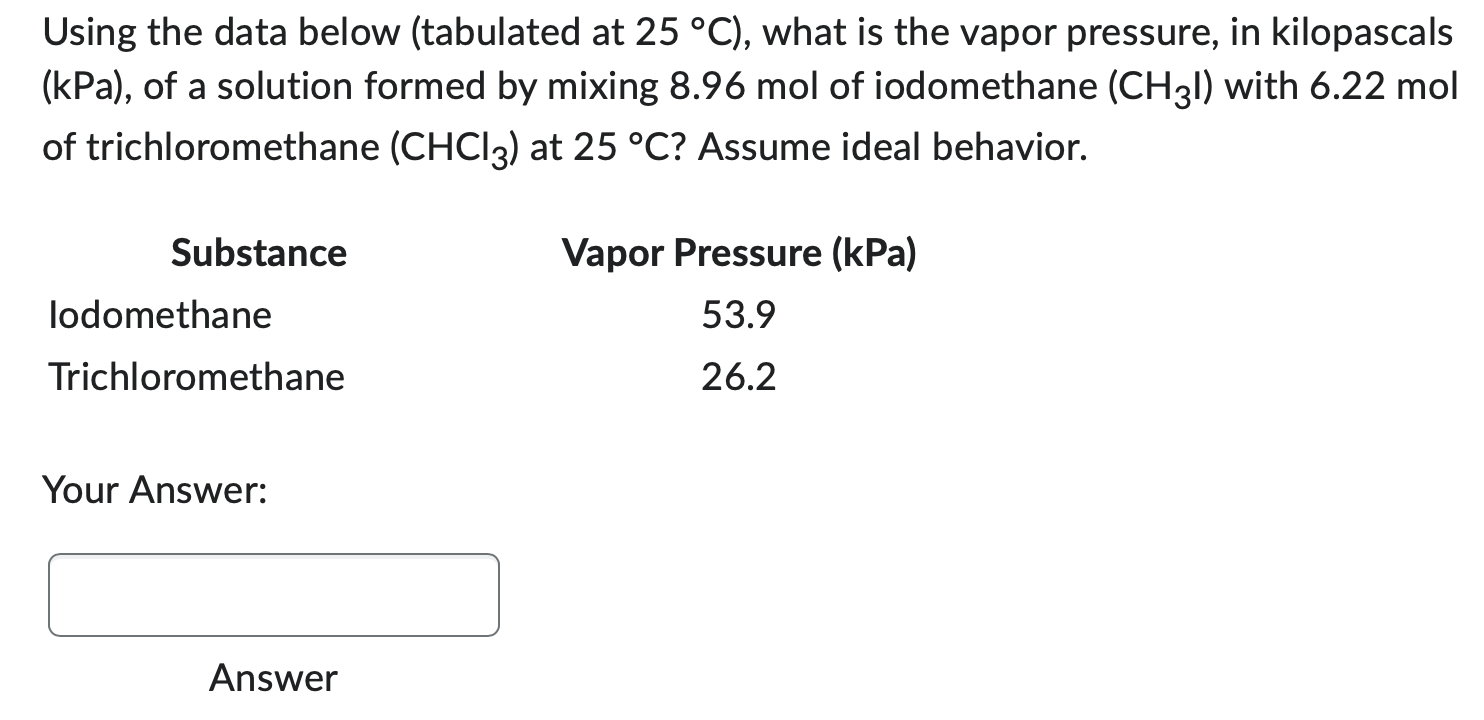
Using the data below (tabulated at 25C ), what is the vapor pressure, in kilopascals (kPa), of a solution formed by mixing 8.96mol of iodomethane (CH3l) with 6.22mol of trichloromethane (CHCl3) at 25C ? Assume ideal behavior. Your
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


