Question: Using the energies for various interactions given in the table below, draw an energy vs. dihedral angle plot for 2-methylbutane. Draw a Newman projection looking
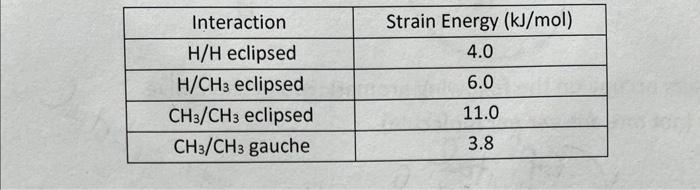
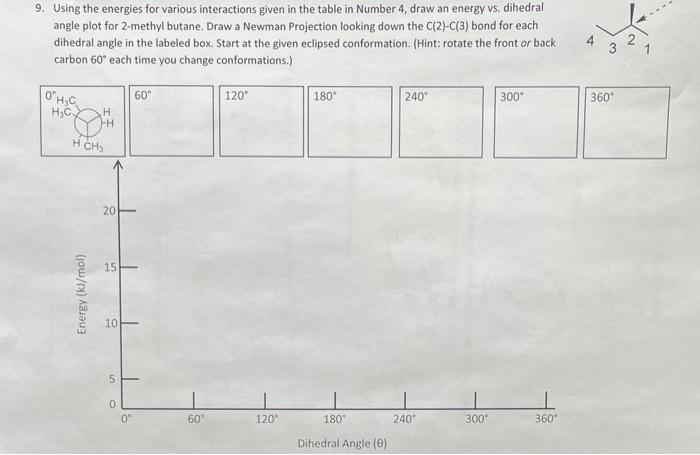
\begin{tabular}{|c|c|} \hline Interaction & Strain Energy (kJ/mol) \\ \hline H/H eclipsed & 4.0 \\ \hline H/CH3 eclipsed & 6.0 \\ \hline CH3/CH3 eclipsed & 11.0 \\ \hline CH3/CH3 gauche & 3.8 \\ \hline \end{tabular} 9. Using the energies for various interactions given in the table in Number 4 , draw an energy vs. dihedral angle plot for 2-methyl butane. Draw a Newman Projection looking down the C(2)C(3) bond for each dihedral angle in the labeled box. Start at the given eclipsed conformation. (Hint: rotate the front or back carbon 60 each time vou change conformations.I
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


