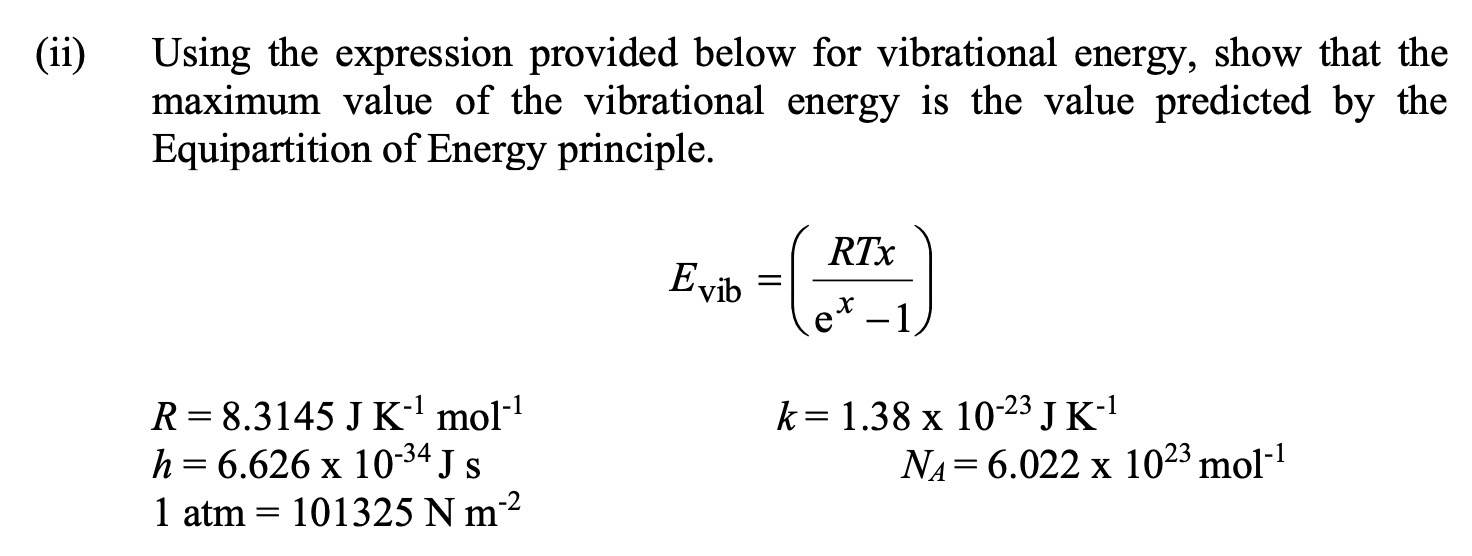
Question: Using the expression provided below for vibrational energy, show that the maximum value of the vibrational energy is the value predicted by the Equipartition of
Using the expression provided below for vibrational energy, show that the maximum value of the vibrational energy is the value predicted by the Equipartition of Energy principle.
marks
marks
R J K mol h x J s
atm N m
RTx Evib x e
k x J K
NA x molii Using the expression provided below for vibrational energy, show that the
maximum value of the vibrational energy is the value predicted by the
Equipartition of Energy principle.
atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


