Question: Using the first two data sheets, can someone help me answer the last page for calculations Data Sheet Literature value for the freezing point of
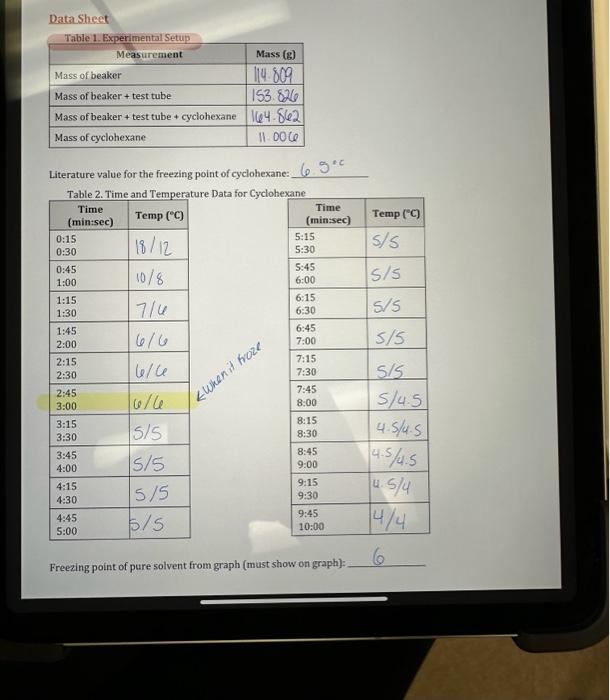
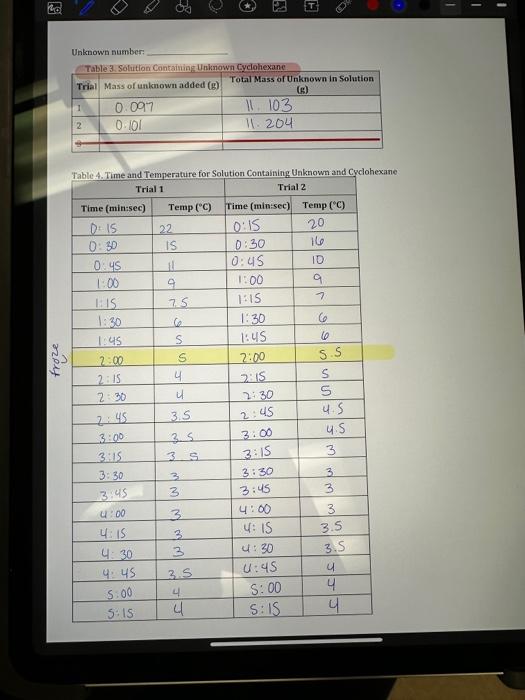

Data Sheet Literature value for the freezing point of cyclohexane: 6.5 Tabin a tima and Tammarstare Data for Cyclohavane Freezing point of pure solvent from graph (must show on graph): 6 Table 4. Time and Temperature for Solution Containing Unknown and Cyclohexane 1. Mass of beaker, test tube (g)=15.8.809 2. Freezing point, from cooling curve (C) Circle plateau on graph. =6C B. Freezing Point of Cyclohexane plus Unknown Solute: 1. Mass of beaker, test tube, cyclohexane (g)=164.862 2. Mass of cyclohexane: =11.006 3. Mass of added solute (g)=0.198 4. Freezing point, from cooling curve (C)=SC Circle on graph where lines intersect - identify graphs. CALCULATIONS On separate paper: (Please re-write the steps down when you show calculations, you must show calculations step by step) Show formula Insert #s Show answer. 2. Freezing point change, Tr(C)=TtrolventTfsolution Note: Treolvent \& Trsolution come from Graphs! Tfwent comes from Plateau. Trsolution comes from intersection - circle it and show it on graph. 3. Mass of cyclohexane in solution: gX(1kg/1000g)= Kg 4. Moles of solute: Tr=km Ti=kr(molsolute)kgsolventmol=(Ti)(kgsolvent)kr1,20.0Ckg/mol 5. Mass of solute in solution, TOTAL = 6. Molar mass of solute = Calc 5/Calc4(g/mol) 7. Average molar mass of solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


