Question: Using the following equations, work out how much O_(2) in m(g)/(m)L A. Converting drops to m(g)/(L) : B. Converting O_(2)% Saturation to O_(2)(m(g)/(L))
Using the following equations, work out how much
O_(2)in
m(g)/(m)L\ A. Converting "drops" to
m(g)/(L):\ B. Converting
O_(2)%Saturation to
O_(2)(m(g)/(L))\
DO((mg)/(L))=(A_(drops ))/(2)\
DO(((mg)/(L))/(O_(2)%))=(DO% bubbled water ((mg)/(L)))/(100%) 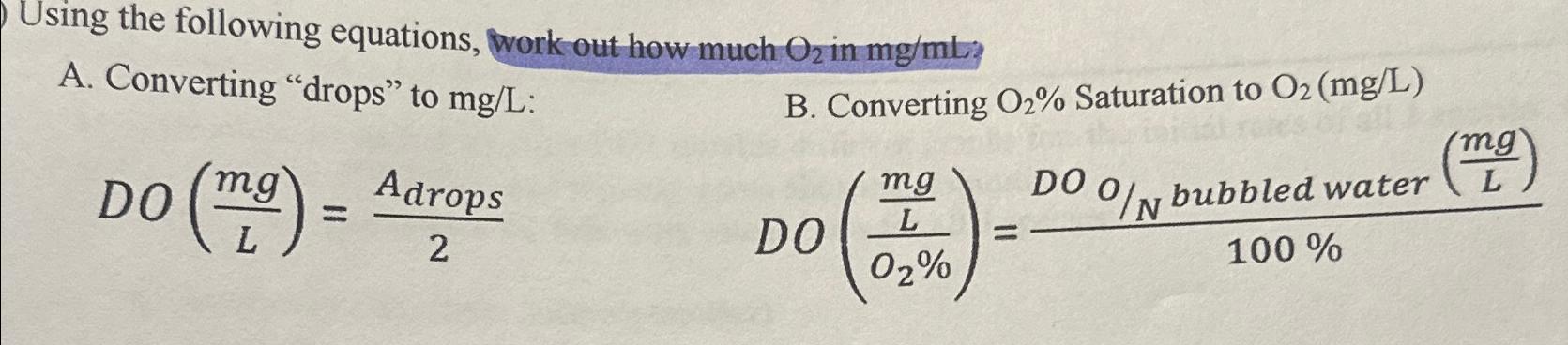
Using the following equations, work out how much O2 in mg/mL A. Converting "drops" to mg/L : B. Converting O2% Saturation to O2(mg/L) DO(Lmg)=2Adrops DO(O2%Lmg)=100%DOONbubbledwater(Lmg) Using the following equations, work out how much O2 in mg/mL A. Converting "drops" to mg/L : B. Converting O2% Saturation to O2(mg/L) DO(Lmg)=2Adrops DO(O2%Lmg)=100%DOONbubbledwater(Lmg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


