Question: Using the information below, calculate the equilibrium constant, at 900K, for the reaction: The F atom ground state is 4-fold degenerate. The next highest electronic
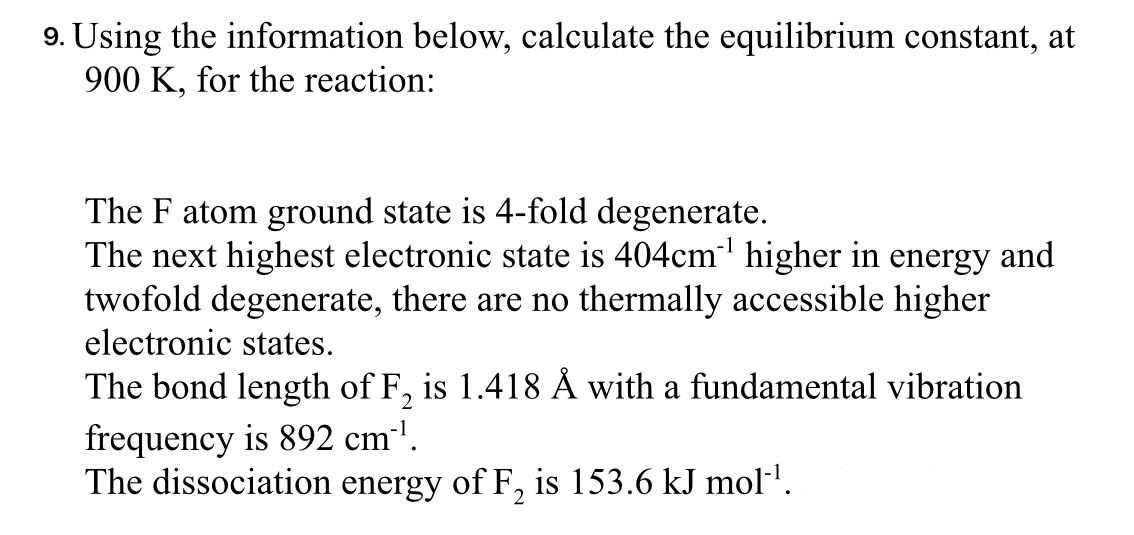
Using the information below, calculate the equilibrium constant, at 900K, for the reaction: The F atom ground state is 4-fold degenerate. The next highest electronic state is 404cm1 higher in energy and twofold degenerate, there are no thermally accessible higher electronic states. The bond length of F2 is 1.418A with a fundamental vibration frequency is 892cm1. The dissociation energy of F2 is 153.6kJmol1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


