Question: Using the information from part 3, complete part b and c, along with questions 4 and 5. Thank you so much! 3. a. An aqueous


Using the information from part 3, complete part b and c, along with questions 4 and 5. Thank you so much!
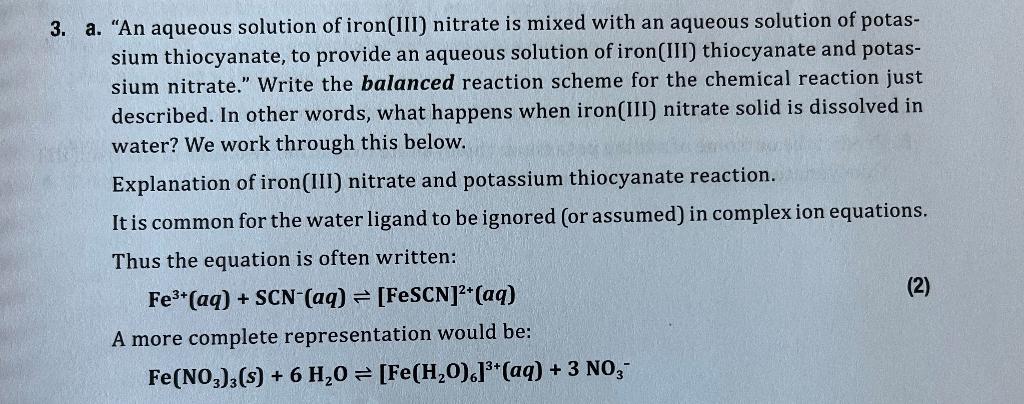
3. a. "An aqueous solution of iron(III) nitrate is mixed with an aqueous solution of potassium thiocyanate, to provide an aqueous solution of iron(III) thiocyanate and potassium nitrate." Write the balanced reaction scheme for the chemical reaction just described. In other words, what happens when iron(III) nitrate solid is dissolved in water? We work through this below. Explanation of iron(III) nitrate and potassium thiocyanate reaction. It is common for the water ligand to be ignored (or assumed) in complex ion equations. Thus the equation is often written: Fe3+(aq)+SCN(aq)[FeSCN]2+(aq) A more complete representation would be: Fe(NO3)3(s)+6H2O[Fe(H2O)6]3+(aq)+3NO3 And [Fe(H2O)6]3+(aq)+KSCN(aq)[Fe(H2O)5SCN]2+(aq)+H2O(I)+K+ or an overall complete reaction of: Fe(NO3)3(aq)+KSCN(aq)[FeSCN]2+(aq)+K+(aq)+3NO3(aq) Finally we could include the anions with the cations: [Fe(H2O)6](NO3)3(aq)+KSCN(aq)[Fe(H2O)5SCN](NO3)2(aq)+H2O(l)+KNO3(aq) Thus we see that Eq. (1) is often the most useful representation. Equation (2) indicates a more complete understanding of the complexes and the red species. As more SCNis added the equilibrium in Eq. (2) will shift to substitute more SCNfor H2O in the compound. b. Why do six waters attach to the iron(III) ion? c. Why does one SCN(aq) ion displace one water? Why don't more SCN(aq) ions replace more waters? (5) 4. Predict the outcome of adding iron(III) nitrate to the test tube from reaction (2) above. 5. Predict the outcome of adding potassium thiocyanate to another test tube of the iron(III) thiocyanate solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


