Question: Using the information provided, answer the following questions. For full credit, show all calculations and account for significant figures. Consider the combustion reaction for glucose
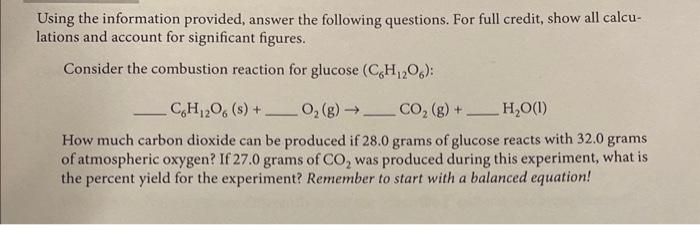
Using the information provided, answer the following questions. For full credit, show all calculations and account for significant figures. Consider the combustion reaction for glucose (C6H12O6) : C6H12O6(s)+O2(g)CO2(g)+H2O(l) How much carbon dioxide can be produced if 28.0 grams of glucose reacts with 32.0 grams of atmospheric oxygen? If 27.0 grams of CO2 was produced during this experiment, what is the percent yield for the experiment? Remember to start with a balanced equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


