Question: Using the provided table and the equation below, determine the heat of formation (in kJ/mol) for CuBr. CuCl (s) + Br(l) CuBr (s) + Cl
Using the provided table and the equation below, determine the heat of formation (in kJ/mol) for CuBr. CuCl (s) + Br(l) CuBr (s) + Cl (g) H = 64.1 kJ/mol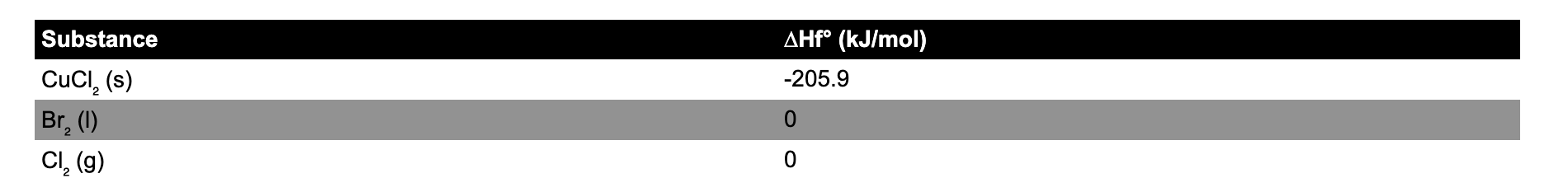
\begin{tabular}{ll} Substance & Hf ( kJ/mol) \\ \hline CuCl2(s) & 205.9 \\ Br2(l) & 0 \\ Cl2(g) & 0 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


