Question: Using the same container as for the last two questions, assume that N=50 molecules are initially trapped on the left hand side. The gas then
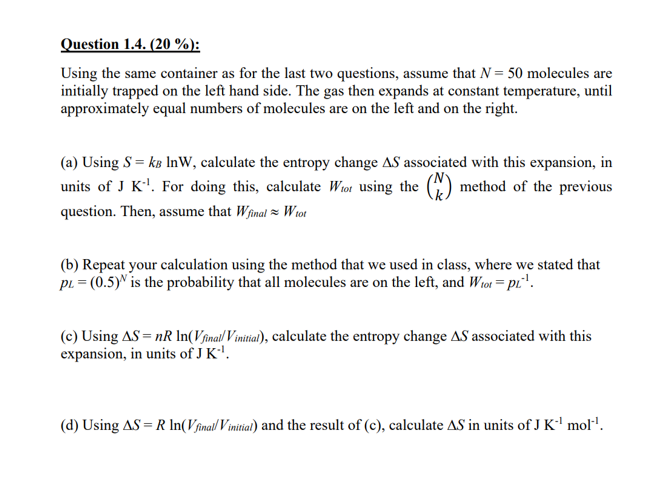
Using the same container as for the last two questions, assume that N=50 molecules are initially trapped on the left hand side. The gas then expands at constant temperature, until approximately equal numbers of molecules are on the left and on the right. (a) Using S=kBlnW, calculate the entropy change S associated with this expansion, in units of JK1. For doing this, calculate Wtot using the (Nk) method of the previous question. Then, assume that WfinalWtot (b) Repeat your calculation using the method that we used in class, where we stated that pL=(0.5)N is the probability that all molecules are on the left, and Wtot=pL1. (c) Using S=nRln(Vfinal/Vinitial), calculate the entropy change S associated with this expansion, in units of JK1. (d) Using S=Rln(Vfinal/Vinitial) and the result of (c), calculate S in units of JK1mol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


