Question: Using the table below (from Burney and Harbour), determine the half-cell reaction potential for E(Np/Np4+) (i.e., the conversion of neptunium from Np to Np4+ STANDARD
- Using the table below (from Burney and Harbour), determine the half-cell reaction potential for E(Np/Np4+) (i.e., the conversion of neptunium from Np to Np4+
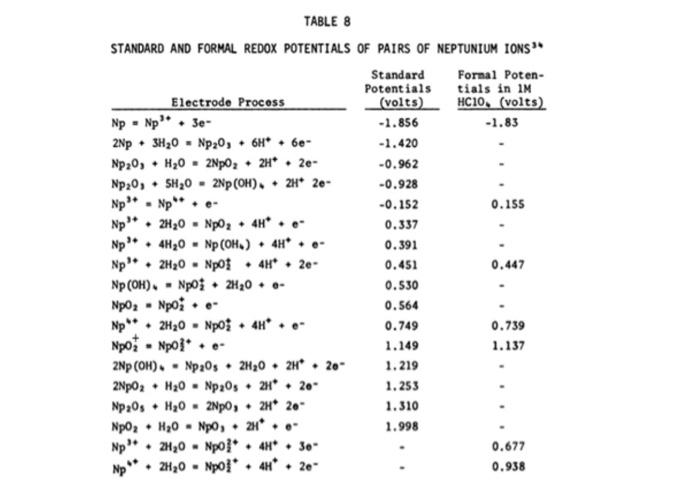
STANDARD AND FORMAL REDOX POTENTIALS OF PAIRS OF NEPTUNIUM IONS
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


